What Is The Forecast Growth Rate For The Guillain-Barre Syndrome Drugs Market?
The Business Research Company’s market reports offer an in-depth analysis on the market’s growth potential, major drivers, key trends and more.
Rapid Market Expansion in Recent Years
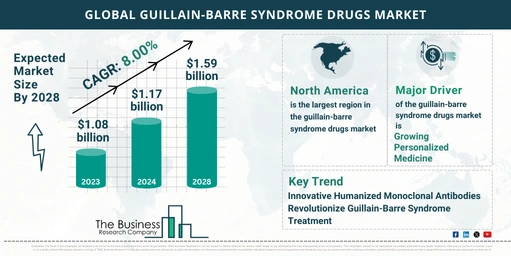
- Market Growth: The Guillain-Barre Syndrome (GBS) drugs market has experienced significant growth, increasing from $1.08 billion in 2023 to $1.17 billion in 2024, with a compound annual growth rate (CAGR) of 7.8%.
- Contributing Factors: This growth can be attributed to advancements in research and development, governmental support, and a heightened focus on neurology and autoimmune diseases.
- Healthcare Demand: The rise in demand for hospital and clinic services has further driven the market’s expansion.
Future Market Projections
- Forecasted Growth: The GBS drugs market is expected to continue its strong upward trajectory, reaching $1.59 billion by 2028 at a CAGR of 8.0%.
- Drivers of Growth: Key factors include an increasing incidence of GBS, a growing geriatric population, and rising demand for oral and parenteral medications.
- Pharmacy Impact: The growing role of hospital and retail pharmacies will play a crucial part in this expansion.
Read More On The Guillain-Barre Syndrome Drugs Market Report 2024 – https://www.thebusinessresearchcompany.com/report/guillain-barre-syndrome-drugs-global-market-report
Personalized Medicine: A Key Growth Driver
- Tailored Treatments: Personalized medicine, which tailors treatments based on individual characteristics such as genetics, is becoming increasingly important in GBS treatment.
- Regulatory Support: Regulatory bodies are recognizing the potential of personalized therapies, as seen in the FDA’s approval of 16 novel personalized therapies for rare diseases in 2023.
- Improved Outcomes: By tailoring treatments to individual patient profiles, personalized medicine aims to enhance therapeutic outcomes and reduce adverse effects.
Innovations in Humanized Monoclonal Antibodies
- Advanced Therapies: Leading pharmaceutical companies are developing humanized monoclonal antibodies to revolutionize GBS treatment.
- Targeted Treatment: These antibodies target specific components of the immune system involved in GBS, offering more precise and effective therapies.
- Recent Developments: Annexon Inc.’s ANX005, a humanized monoclonal antibody, has received orphan drug designation from both the EMA and FDA. The drug shows promise in reducing inflammation and nerve damage in GBS patients.
Strategic Acquisitions Bolster Market Position
- Grifols SA Acquisition: In 2022, Grifols SA acquired Biotest AG for $1.17 billion, a strategic move to expand its plasma supply and strengthen its market presence.
- Global Reach: This acquisition enhances Grifols’ operations in Europe, the Middle East, and Africa, and supports the global availability of plasma-derived therapies.
- Collaborative Efforts: The partnership between Grifols and Biotest aims to advance the treatment options for GBS and improve patient outcomes.
Market Segmentation and Regional Growth
- Drug Class Segmentation: The market is segmented into immunoglobulins (IVIG), corticosteroids, and plasma exchange.
- Treatment Type: It includes first-line treatments and adjunctive or supportive treatments.
- Regional Insights: North America was the largest region in the GBS drugs market in 2023, with Asia-Pacific expected to be the fastest-growing region during the forecast period.
Conclusion
The Guillain-Barre Syndrome drugs market is set for continued growth, driven by advancements in personalized medicine, innovative therapies like humanized monoclonal antibodies, and strategic acquisitions. With a strong focus on improving patient outcomes and expanding treatment options, the market will likely see significant developments in the coming years.
Request for A Sample Of The Global Guillain-Barre Syndrome Drugs Market Report:
https://www.thebusinessresearchcompany.com/sample_request?id=15581&type=smp