Key Drivers and Opportunities in the Balloon Valvuloplasty Device Market: Insights into Trends and Growth
Updated 2025 Market Reports Released: Trends, Forecasts to 2034 – Early Purchase Your Competitive Edge Today!
What Long-Term Growth Rate is Expected for the Balloon Valvuloplasty Device Market Between 2025 and 2034?
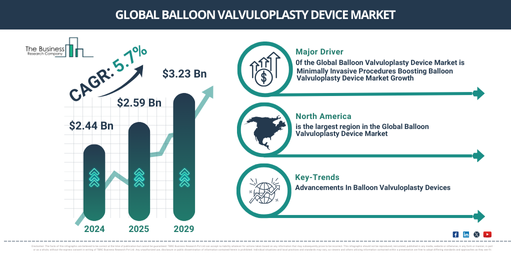
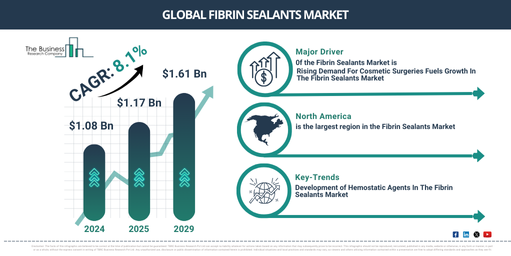
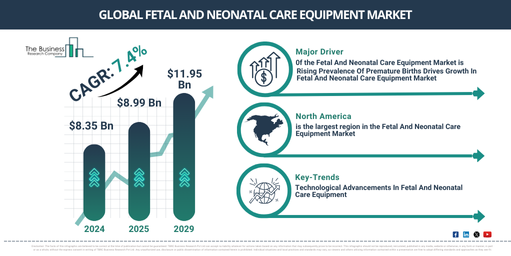
In recent times, the market size for balloon valvuloplasty devices has experienced substantial growth. The market is projected to rise from $2.44 billion in 2024 to $2.59 billion in 2025, reflecting a compound annual growth rate (CAGR) of 6.0%. Factors contributing to this growth during the historic period include an increase in healthcare spending, development of healthcare infrastructure, enhanced awareness and diagnostic abilities, a preference for outpatient environments, the proliferation of telemedicine for improved access, and a focus on enhancing patients’ quality of life.
In the coming years, the market size for balloon valvuloplasty devices is anticipated to experience robust expansion. By 2029, it is projected to reach a value of $3.23 billion with a Compound Annual Growth Rate (CAGR) of 5.7%. The predicted growth in the forecast period can be linked to a variety of factors, including a rise in heart valve disease amongst elderly populations globally, an increased preference for less invasive treatment options for heart valve issues, quick approval and clearance for new balloon valvuloplasty devices, and an emphasis on affordable healthcare solutions. Some noteworthy trends during this period are improved imaging methods for accurate diagnoses, personalized treatment strategies, escalated investment in research and development, the advent of regenerative approaches in managing cardiovascular diseases, and the incorporation of digital health technologies.
What Industry-Specific Factors Are Fueling the Growth of the Balloon Valvuloplasty Device Market?
The growing preference for minimally invasive procedures is anticipated to boost the expansion of the balloon valvuloplasty device market. As these medical techniques are performed through tiny incisions or natural body openings, they minimize trauma and encourage speedy recovery compared to conventional surgeries. The surge in demand for minimally invasive procedures is attributed to factors such as shorter recovery periods, reduced risk of complications, and less post-operative discomfort as opposed to traditional surgeries. Balloon valvuloplasty devices find their application in these procedures, efficiently dilating narrow heart valves and enabling improved blood circulation, thus alleviating symptoms. For instance, in January 2024, US-based Intuitive Surgical Inc., a producer of robotic products, recorded that they had distributed 415 da Vinci surgical systems (a robotic surgical system employing a minimally invasive surgical method) in the fourth quarter of 2023, marking a 12% growth compared to 2022. Hence, the surging preference for minimally invasive procedures is fueling the balloon valvuloplasty device market’s expansion.
Get Your Free Sample of the Global Balloon Valvuloplasty Device Market Report Now!
https://www.thebusinessresearchcompany.com/sample.aspx?id=17090&type=smp
What Are the Key Firms That Are Driving Transformation in the Balloon Valvuloplasty Device Market?
Major companies operating in the balloon valvuloplasty device market are:
• Cardinal Health Inc._x000D_
• Abbott Laboratories_x000D_
• Medtronic plc_x000D_
• Philips Healthcare_x000D_
• Becton Dickinson Company_x000D_
What Current Trends in the Balloon Valvuloplasty Device Market Should Industry Players Pay Attention To?
Significant enterprises in the balloon valvuloplasty device market are working towards introducing cutting-edge products such as Pacing Guidewires to set themselves apart in the competitive landscape. Pacing Guidewires provide a crucial function of temporary cardiac pacing during the procedure, which ensures the heart keeps a steady rhythm while the balloon catheter is being inserted and inflated to widen the contracted valve. To exemplify, Teleflex Incorporated, a medical device corporation based in the US, was given clearance for the Wattson Temporary Pacing Guidewire by the Food and Drug Administration (FDA) in June 2023. This innovative device has been exclusively created for use in transcatheter aortic valve replacement (TAVR) and balloon aortic valvuloplasty (BAV) procedures. The Wattson Temporary Pacing Guidewire provides dual functionality in terms of both supporting valve delivery and enabling ventricular bipolar pacing during these structural heart operations. It is fashioned with a flexible distal pigtail shape, multiple electrodes, and a bipolar design, all aimed at minimising the risk of ventricular perforation and ensuring dependable electrical capture during rapid pacing.
Get Instant Access to the Global Balloon Valvuloplasty Device Market Report with Swift Delivery!
https://www.thebusinessresearchcompany.com/report/balloon-valvuloplasty-device-global-market-report
What Are the Key Components of the Balloon Valvuloplasty Device Market, and How Do Its Segments Perform?
The balloon valvuloplasty device market covered in this report is segmented –
1) By Product Type: Standard Balloon, Cutting Balloon, Scoring Balloon, High-Pressure Balloon, Low-Pressure Balloon
2) By Age Group: Pediatric, Adult
3) By End-use: Hospitals, Ambulatory Surgical Centers, Specialty Clinics, Other End Uses
Subsegments:
1) By Standard Balloon: Non-Compliant Balloons, Compliant Balloons
2) By Cutting Balloon: Single-Cutting Balloon, Multi-Cutting Balloon
3) By Scoring Balloon: Single-Score Balloons, Dual-Score Balloons
4) By High-Pressure Balloon: Non-Compliant High-Pressure Balloons, Compliant High-Pressure Balloons
5) By Low-Pressure Balloon: Semi-Compliant Low-Pressure Balloons, Non-Compliant Low-Pressure Balloons
What Regions Are Driving Expansion in the Balloon Valvuloplasty Device Market?
North America was the largest region in the balloon valvuloplasty device market in 2023. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the balloon valvuloplasty device market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
What Key Parameters Define the Balloon Valvuloplasty Device Market’s Scope?
A balloon valvuloplasty device is a medical instrument used to treat heart valve stenosis by inserting a balloon into the narrowed valve and inflating it to widen the opening, improving blood flow. This non-surgical procedure is minimally invasive and aims to relieve symptoms such as chest pain and shortness of breath, enhancing cardiac function and patient quality of life.
Browse Through More Similar Reports By The Business Research Company:
Drug Eluting Balloons Catheters Global Market Report 2024
ENT Devices Global Market Report 2024
https://www.thebusinessresearchcompany.com/report/ent-devices-global-market-report
Orthopedic Devices Global Market Report 2024
https://www.thebusinessresearchcompany.com/report/orthopedic-devices-global-market-report
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Contact us at:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at info@tbrc.info
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
Found this article helpful? Share it on: