Feiba NF Market Trends 2025-2034: Insights into Growth and Strategic Opportunities Ahead
Updated 2025 Market Reports Released: Trends, Forecasts to 2034 – Early Purchase Your Competitive Edge Today!
How Will the Feiba NF Market’s Growth Rate Evolve Over the Forecast Period to 2034?
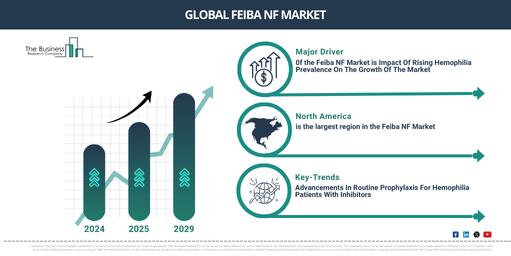
The market for feiba NF has seen a XX% (CAGR) surge in recent years. A growth from $XX million in the year 2024 to $XX million in 2025, representing a compound annual growth rate (CAGR) of XX%, is expected. The historic period’s growth can be credited to the rise in hemophilia cases, a shift towards preventive treatment approaches, a focus on life quality, and advancements in production techniques.
Anticipations are high for the Feiba NF market, with size projections indicating a growth rate (CAGR) of XX over the upcoming years. By 2029, predictions suggest the market will have expanded to a value of $XX million, revealing a compound annual growth rate (CAGR) of XX%. Factors contributing to this promising forecast period include heightened consciousness surrounding the management of hemophilia, regulatory accreditations, introductions of new indications, effectiveness in controlling bleeding incidents, and a heightened occurrence of hemophilia cases. Furthermore, the market’s penetration into developing economies is set to boost its progress. Over the future duration, key trends expected to shape the market include product advancements, strategic collaborations, and product endorsements.
What Key Drivers Are Accelerating the Growth of the Feiba NF Market During the Forecast Period?
The escalating frequency of hemophilia is projected to propel the feiba NF market’s expansion. Hemophilia is an uncommon hereditary blood disorder characterized by the inability of blood to clot correctly, leading to excessive bleeding during injuries or surgeries and spontaneous internal bleeding. The increase in hemophilia cases is attributed to factors such as enhanced genetic testing, burgeoning awareness and diagnosis, emergent genetic mutations, and advanced treatment methodologies that result in longer lifecycles. FEIBA NF assists hemophilia patients by offering an efficient treatment to manage bleeding episodes and routine prophylaxis, thereby reducing the occurrence of such episodes and bettering long-term care. This ultimately enriches the patient’s life quality and mitigates the risks associated with hemophilia. For example, as per the National Library of Medicine, a US-based biomedical library, in June 2023, hemophilia is prevalent in about 1 in 10,000 live births, affecting nearly 400,000 individuals worldwide. Hemophilia A is more frequent, representing 80-85% of cases, and occurring in 1 in 5,000 live male births, while Hemophilia B occurs in 1 in 30,000 live male births. Hemophilia C is comparatively tighter, triggered by factor XI deficiency, and appears in about 1 in 100,000 people, with a significant incidence among Ashkenazi Jews (around 8%). Moreover, the World Federation of Hemophilia (WFH), a Canada-based non-profit entity, stated that as of June 2023, the estimated prevalence of hemophilia A, as of 2022, was around 17.1 per 100,000 males for all cases and 6.0 per 100,000 males for severe hemophilia A. For hemophilia B, the rates were 3.8 per 100,000 males overall, and 1.1 per 100,000 males for severe instances. Consequently, the escalating prevalence of hemophilia is stimulating the feiba NF market’s growth.
Get Your Free Sample of the Global Feiba NF Market Report Now!
https://www.thebusinessresearchcompany.com/sample.aspx?id=20074&type=smp
Who Are the Influential Players Reshaping the Feiba NF Market Landscape?
Major companies operating in the feiba nf market include Takeda Pharmaceuticals Inc.
What New and Evolving Trends Are Having a Lasting Impact on the Feiba NF Market?
Technological innovations, focused specifically on routine prophylaxis, serve as the major development in the feiba NF market in order to decrease the frequency of bleeding episodes in hemophilia patients with inhibitors. Routine prophylaxis, a type of preventive care focused on minimizing disease and infection risks through methods such as vaccinations, health screenings, or pre-procedural medications. An illustrative example occurred in June 2023 when Takeda Pharmaceuticals U.S.A. Inc., a biopharmaceutical company based in the United States, received FDA approval for FEIBA. This product is not only purposed for controlling bleeding episodes but also for providing routine prophylaxis to control or lessen the frequency of bleeding episodes in patients suffering from hemophilia with inhibitors. This progression magnifies its function in the persistent handling of hemophilia, presenting a more preemptive system for patients to manage their health. The implementation of routine prophylaxis marks a major progression in the treatment of hemophilia patients with inhibitors as its helps to decrease bleeding episodes, therefore, enhancing the overall quality of life.
Pre-order Your Report for Quick and Easy Delivery!
https://www.thebusinessresearchcompany.com/report/feiba-nf-global-market-report
Which Key Segments Stand Out in Understanding the Composition of the Feiba NF Market?
The feiba nf market covered in this report is segmented –
1) By Indication: Hemophilia A and B with inhibitors, Prophylactic treatment, On-demand treatment
2) By Distribution Channel: Hospitals, Specialty Pharmacies, Direct-to-consumer
3) By End User: Adult, Pediatric
Which Geographic Locations Are Critical to the Growth of the Feiba NF Market?
North America was the largest region in the feiba NF market in 2024. The regions covered in the feiba nf market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa.
What Are the Key Characteristics That Define the Feiba NF Market?
FEIBA NF (Factor VIII Inhibitor Bypassing Activity) is a plasma-derived medication used to manage bleeding in haemophilia patients with inhibitors against factors VIII or IX. It contains coagulation factors that bypass these inhibitors, facilitating clotting. It’s indicated for bleeding episodes and surgical procedures, with careful monitoring for thromboembolic risks.
Browse Through More Similar Reports By The Business Research Company:
Rotavirus Prophylaxis Global Market Report 2025
https://www.thebusinessresearchcompany.com/report/rotavirus-prophylaxis-global-market-report
Cyclic Heavy Menstrual Bleeding Global Market Report 2025
Bleeding Disorders Treatment Global Market Report 2025
https://www.thebusinessresearchcompany.com/report/bleeding-disorders-treatment-global-market-report
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Contact us at:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at info@tbrc.info
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
Found this article helpful? Share it on: