How Will the Pharmaceutical Sterility Testing Market Grow? Key Trends and Opportunities for 2025 and Beyond
Updated 2025 Market Reports Released: Trends, Forecasts to 2034 – Early Purchase Your Competitive Edge Today!
How Will the Pharmaceutical Sterility Testing Market Grow Over the Forecast Period Based on Its Expected CAGR?
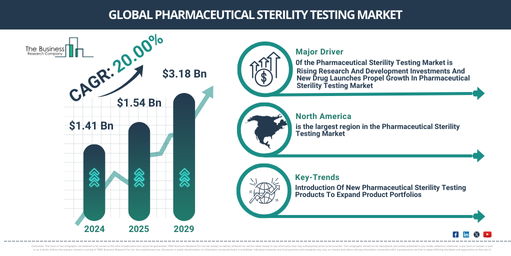
The size of the pharmaceutical sterility testing market has seen a significant expansion in recent years. The market is projected to escalate from $1.41 billion in 2024 to $1.54 billion in 2025, showcasing a compound annual growth rate (CAGR) of 9.0%. The historic growth is due to increased demand for superior quality pharmaceutical products, an enhanced focus on drug safety and efficacy, a rise in the requirement for biologics and biosimilar drugs, a higher incidence of infectious diseases, and an increase in life science research investments.
The market for sterility testing in the pharmaceutical industry is projected to experience accelerated growth in the forthcoming years, with the market size expected to reach $3.18 billion in 2029 at a compound annual growth rate (CAGR) of 20.0%. This anticipated growth in the forecast period can be credited to the rise in biopharmaceuticals, personalized medicine, increasing trend of outsourcing, focus on quality assurance, and preparedness for pandemics. Key trends to watch out during the forecast period include accelerated microbiological methods, combined products, data integrity and digitization, automation and robotics, and cutting-edge analytical techniques.
How Are the key drivers Contributing to the Expansion of the Pharmaceutical Sterility Testing Market?
The pharmaceutical sterility testing market’s growth has been significantly influenced by a surge in drug launches and increased investment in research and development (R&D). Sterility, which ensures that drugs are free from viable microorganisms and product contamination, is a crucial phase in drug manufacturing. For example, Pharmaceutical Technology, a US-based independent resource for bio/pharmaceutical formulation knowledge and analysis, published an article in March 2022 revealing the UK government’s commitment of $347 million (£260 million) to foster R&D and create innovative treatments, tools, and diagnostics. IQVIA, a US-based firm offering advanced analytics, technology solutions, and clinical research services, declared in a December 2021 report that we could expect around 300 new drugs to hit the market by 2026. This prediction surpasses the average number of drugs launched within the past decade, with a shift anticipated towards specialty, niche, and orphan drugs. The report also projected a $196 billion increase in new product spending over the next five years, which will be marginally balanced by a $188 billion reduction in brand spending due to loss of exclusivity.
Get Your Free Sample of the Global Pharmaceutical Sterility Testing Market Report Now!
https://www.thebusinessresearchcompany.com/sample.aspx?id=2826&type=smp
Who Are the Dominant Players Expanding Their Reach in the Pharmaceutical Sterility Testing Market?
Major companies operating in the pharmaceutical sterility testing market include Pacific Biolabs Inc., STERIS Corporation, Boston Analytical Inc., Gibraltar Laboratories Inc., Sartorius AG, Solvias AG, SGS S.A., Toxikon Inc., Pace Analytical Services LLC, Charles River Laboratories International Inc., Nelson Laboratories Inc., Rapid Micro Biosystems Inc., bioMérieux Inc., Merck KGaA, WuXi AppTec, Sotera Health LLC, Dynalabs LLC, Infinity Laboratories, Eurofins Scientific SE, Avomeen Analytical Services, Microbac Laboratories Inc., Analytical Lab Group LLC, North American Science Associates Inc., Medical Device Testing Services Inc., Microtest Laboratories Inc., BioScreen Testing Services Inc., Microchem Laboratory Inc., Bactolac Pharmaceutical Inc., Micro Quality Labs Inc., Boston Microfluidics Inc., BioPharma Services Inc., Microbiology Research Associates, BioScience Laboratories Inc.
What Key Trends Are Currently Impacting the Pharmaceutical Sterility Testing Market’s Development?
Firms in the pharmaceutical testing market are debuting innovative sterility testing products and services to augment their offerings and broaden their market presence. These companies are rolling out technologically advanced solutions and well-prepared services to leverage the growing pharmaceutical sterility testing market’s potential. For example, STEMart, an American company that offers integrated medical device CRO services, initiated an extensive range of microbiology and sterility testing for sterile, non-pyrogenic products in June 2022. Comprehensive microbiology and sterility testing encompasses several services, including the Antibiotic Potency Test intended to assess the bioactivity or efficacy of specific antibiotics for medical devices.
Get Instant Access to the Global Pharmaceutical Sterility Testing Market Report with Swift Delivery!
Which Market Segments Are Driving Strategic Growth and Trends in the Pharmaceutical Sterility Testing Market?
The pharmaceutical sterility testing market covered in this report is segmented –
1) By Sample: Sterile Drugs, Medical Devices, Biologics and Therapeutics
2) By Product Type: Instruments, Kits and Reagents, Services
3) By Type: In- house, Outsourcing
4) By Test Type: Sterility Testing, Bioburden Testing, Bacterial Endotoxin Testing
5) By End- User: Compounding Pharmacies, Medical Devices Companies, Pharmaceutical Companies
Subsegments:
1) By Sterile Drugs: Injectable Drugs, Infusion Solutions, Ophthalmic Solutions
2) By Medical Devices: Surgical Instruments, Implants, Catheters
3) By Biologics And Therapeutics: Vaccines, Gene Therapies, Cell Therapies
Which Regions Are Setting the Pace for Pharmaceutical Sterility Testing Market Growth?
The countries covered in the pharmaceutical sterility testing market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Italy, Spain, Canada.
What Are the Defining Features of the Pharmaceutical Sterility Testing Market?
Pharmaceutical sterility testing refers to a test that is intended to show if biological parenteral made for human use contain extraneous, viable contaminating microorganisms or not. All pharmaceutical items used on patients by medical professionals must be of the highest quality and extremely safe. Otherwise, it could harm the patients. Testing for sterility assures that the offered product is suitably sterile.
Browse Through More Similar Reports By The Business Research Company:
Pharmaceutical Contract Development And Manufacturing Organization (CMO) Global Market Report 2024
Pharmaceutical Excipients Global Market Report 2024
https://www.thebusinessresearchcompany.com/report/pharmaceutical-excipient-global-market-report
Pharmaceutical Packaging Equipment Global Market Report 2024
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Contact us at:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at [email protected]
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
Found this article helpful? Share it on: