Future Outlook of the Medical Devices Vigilance Market: Growth, Trends, and Emerging Opportunities Explored
Updated 2025 Market Reports Released: Trends, Forecasts to 2034 – Early Purchase Your Competitive Edge Today!
How has the Medical Devices Vigilance market size evolved in recent years?
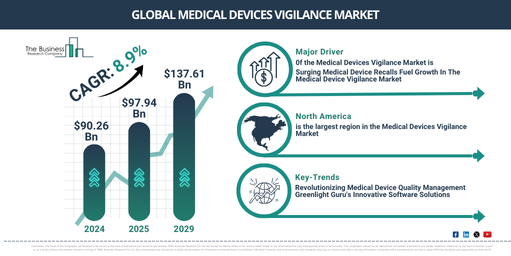
In recent times, there has been notable growth in the market size of medical devices vigilance. It is projected that this market will surge from $90.26 billion in 2024 to $97.94 billion in 2025, exhibiting a compound annual growth rate (CAGR) of 8.5%. The growth witnessed in the historic period can be traced back to several factors. These include increasing knowledge about medical device vigilance, heightened consciousness of its benefits among doctors and patients, growing pressure on the manufacturers of medical devices, a surge in government initiatives, and an escalation in the use of medical devices.
What are the predictions for the medical drone delivery services market size in the coming years?
The market size for medical device vigilance is projected to experience robust growth in the coming years. By 2029, it is estimated to reach $137.61 billion, with a compound annual growth rate (CAGR) of 8.9%. This growth during the forecast period can be tied to a number of factors, such as the rise in recall systems launches for medical devices, an uptick in post market surveillance programs for medical devices, an increasing count of reported adverse events, a growing demand for therapeutics and surgical procedures, and increased intricacy in patient safety regulations. Notable trends during the forecast period encompass technological advancements, the international medical device regulators forum (IMDRF) framework, improved cross-border safety information exchange, remote patient monitoring devices, 3D printing, and custom-made devices.
Get your medical devices vigilance market report here!
https://www.thebusinessresearchcompany.com/report/medical-devices-vigilance-global-market-report
What key factors are fueling the growth of the medical devices vigilance market?
The medical device vigilance market’s future expansion is predicted to be fueled by a rising number of medical recalls. When medical products present risks to public health or violate regulatory standards, medical recalls are the procedures implemented by regulatory bodies, manufacturers, or distributors to delete or amend these items. Medical device vigilance is the ongoing surveillance of commercialized medical devices to uncover adverse incidents, mechanical failures, or safety issues. These findings are notified to regulatory agencies and could prompt further scrutiny and even trigger a product recall if necessary. For example, the American federal agency, the Food and Drug Administration (FDA) noticed an escalation in medical device recalls in December 2022. There were 442 recorded recalls in 2022, marking an almost 10% increase from the 331 in 2021. Thus, the escalating frequency of medical recalls is facilitating the medical device vigilance market’s growth.
How is the global medical devices vigilance market divided into key segments?
The medical devices vigilance market covered in this report is segmented –
1) By Delivery Mode: On-Demand, On-Premise
2) By Application: Therapeutics, Diagnostics, Surgical, Research, Other Applications
3) By End-User: Original Equipment Manufacturers (OEMs), Clinical Research Organizations (CROs), Business Process Outsourcing (BPO) Firms
Subsegments:
1) By On-Demand: Cloud-Based Solutions, Subscription Services, Remote Monitoring And Reporting
2) By On-Premise: In-House Software Solutions, Local Server Deployments, Customized System Implementations
Get your free sample now – explore exclusive market insights:
https://www.thebusinessresearchcompany.com/sample.aspx?id=14137&type=smp
Who are the key firms paving the way for growth in the medical devices vigilance market?
Major companies operating in the medical devices vigilance market are Johnson & Johnson, Intel Corporation, Oracle Corporation, Medtronic, Siemens Healthineers, RELX Group plc, MasterControl Inc., Laerdal Medical, Numerix, Smithers, Omnify Software Inc., Freyr, Sparta Systems, MDI Consultants Inc., Greenlight Guru, Jama Software, Sarjen Systems Pvt. Ltd, Arena Solutions Inc., Xybion Corporation, ZEINCRO Group, Extedo Gmbh, AssurX Inc., AB-Cube, Panacea Pharma Projects Limited, Qvigilance, General Electric (GE) Healthcare, Philips Healthcare, Baxter International, Stryker Corporation, Boston Scientific
Which trends are expected to transform the medical devices vigilance market?
Leading firms in the medical device vigilance market are introducing cutting-edge products, such as quality management software, to enhance the satisfaction of their customers. A quality management software (QMS) is a structured system that records processes, procedures, and roles necessary for accomplishing quality policies and goals. For example, in December 2022, Greenlight Guru, an American corporation that provides systems and software to medical device firms, introduced a QMS software tailored to assist medical device firms in streamlining their processes, speeding up clinical testing, and expediting the delivery of safer products to the market. The software by Greenlight Guru has been pre-validated according to FDA and ISO best practices, intending to help firms stay compliant with constantly evolving standards. The firm also offers training and certification programs to facilitate career advancement in product development, quality, and regulatory assurance.
Unlock exclusive market insights – purchase your research report now for a swift delivery!
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=14428
What regions are contributing significantly to the growth of the medical devices vigilance market?
North America was the largest region in the medical devices vigilance market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the medical devices vigilance market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
Browse Through More Similar Reports By The Business Research Company:
Temperature Monitoring Devices Global Market Report 2024
Respiratory Monitoring Devices Global Market Report 2024
Respiratory Devices And Equipment (Therapeutic And Diagnostic) Global Market Report 2024
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Contact us at:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at [email protected]
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
Found this article helpful? Share it on: