Yescarta Market Growth Forecast: Exploring Trends and Opportunities for the Next Decade
Get 20% off on Global Market Reports until March 31st! Use code FY25SAVE at checkout.
What fueled the previous growth in the yescarta market?
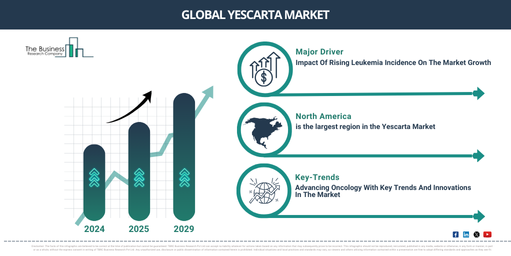
In recent times, the yescarta market size has experienced a XX (HCAGR). It’s projected to expand from $XX million in 2024 to $XX million in 2025, carrying a compound annual growth rate (CAGR) of XX%. This growth during the historic period is driven by the rising acceptance of immuno-oncology treatments, groundbreaking permissions from regulatory authorities, increasing occurrence of blood cancers, advancements in gene therapy technologies, and an increase in healthcare investments in oncology.
What will be the yescarta market size in the future?
The market size of Yescarta is predicted to hit a XX (FCAGR) growth in the upcoming years, eventually reaching a total value of $XX million by 2029, with a Compound Annual Growth Rate (CAGR) of XX%. This projected growth is due to several factors such as, the increasing expansion of forecasts into new cancer indications, advances in manufacturing technologies, and improved healthcare reimbursement for gene therapies. Other contributing factors include greater patient awareness and demand, as well as strategic collaborations in cancer research. Notable trends that will drive this growth include the extension of car T-cell therapies to solid tumors, the creation of next-generation car T-cell therapies, a surging emphasis on individualized cancer treatments, progress in automation for car T-cell production, and the increasing acceptance of combination therapies.
Get your yescarta market report here!
https://www.thebusinessresearchcompany.com/report/yescarta-global-market-report
What main drivers are fueling expansion in the yescarta market?
The progressive rise in leukemia cases is anticipated to stimulate the expansion of the Yescarta market. Leukemia, a cancer affecting the blood and bone marrow, results in an unusual production of white blood cells. This escalating prevalence of leukemia could be attributed to factors like environmental exposure, changes in lifestyle, genetic mutations, and enhanced detection methods. Yescarta, a sophisticated CAR T-cell therapy, transforms leukemia treatment as it allows a patient’s own immune cells to reprogram, target, and eliminate cancerous cells, giving new hope for better outcomes in combating the disease. An example of this is seen in 2024, as reported by the American Cancer Society, a non-profit cancer advocacy organization based in the US. The number of leukemia cases rose to 62,770, showcasing an increase from the 59,610 cases registered in 2023. Thus, the growing prevalence of leukemia propels the Yescarta market.
What key areas define the segmentation of the global yescarta market?
The yescarta market covered in this report is segmented –
1) By Indication: Large B-Cell Lymphoma; Mantle Cell Lymphoma; Acute Lymphoblastic Leukemia; Follicular Lymphoma
2) By Distribution Channel: Hospitals; Specialty Clinics; Online Pharmacies
3) By End User: Adult; Geriatric
Get your free sample now – explore exclusive market insights:
https://www.thebusinessresearchcompany.com/sample.aspx?id=20398&type=smp
Who are the dominant players expanding their reach in the yescarta market?
Major companies operating in the yescarta market are Kite Pharma Inc. ( a subsidiary of Gilead Sciences Inc.)
How are evolving market trends shaping yescarta Strategies?
One prominent trend in the Yescarta market is the emphasis on creating advanced biologics like CAR T-cell therapy, which is intended to increase the effectiveness of treatment, broaden medicinal applications, and improve patient oncology outcomes. CAR T-cell therapy operates by adjusting a patient’s T cells to locate and eradicate cancer cells, providing a custom treatment that may lead to long-term remission and minimized side effects. For instance, in April 2022, Kite Pharma, a biologics manufacturer based in the US, declared that the U.S. Food and Drug Administration (FDA) granted approval to Yescarta (axicabtagene ciloleucel) as the starting treatment for relapsed or refractory large B-cell lymphoma (LBCL). This is the first ever approval of a CAR T-cell therapy for this specific purpose, underscoring the ongoing advancements in cancer treatment, using cutting-edge therapies that harness the body’s immune system to combat cancer more efficiently.
Unlock exclusive market insights – purchase your research report now for a swift delivery!
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=20398
Which regions are emerging as leaders in the yescarta market?
North America was the largest region in the yescarta market in 2024. The regions covered in the yescarta market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
Browse Through More Similar Reports By The Business Research Company:
Chimeric Antigen Receptor T (CAR-T) Cells Global Market Report 2025
Neoantigen Targeted Therapies Global Market Report 2025
https://thebusinessresearchcompany.com/report/neoantigen-targeted-therapies-global-market-report
Prostate Specific Antigen (PSA) Test Global Market Report 2025
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Contact us at:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at [email protected]
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
Found this article helpful? Share it on: