The Future of the CC Chemokine Receptor Type 5 Market: Growth Trends, Market Size, and Opportunities to Watch
2025 Market Reports Update: Market Size Forecasts to 2034, Key Trends, Leading Players, and Top Regions – Get Ahead of the Competition Today!
What Industry-Specific Factors Are Fueling the Growth of theCell And Gene Therapy Clinical Trial Services Market?
The cell and gene therapy clinical trial services market is set to be propelled by the increasing occurrence of genetic diseases. These include conditions arising from variations in an individual’s genetic makeup or DNA. The surge in genetic diseases can be attributed to multiple reasons such as greater accessibility to genetic testing, instances of genetic mutations, and the prominence of marriages between close relatives. To address the mounting prevalence of genetic diseases, cell and gene therapy clinical trial services are promoting the creation of new treatments, adjusting therapies to suit individual patients’ needs, hastening the conversion of research into clinical applications and broadening the choices for patients with genetic disorders. For example, in July 2022, the Cystic Fibrosis (CF) Foundation, a not-for-profit organization based in the United States, reported a hike in the prevalence of people suffering from cystic fibrosis; around 40,000 children and adults in the United States and more than 105,000 people worldwide were affected by the disease in 2022. Thus, the rising occurrence of genetic diseases is fuelling the expansion of the cell and gene therapy clinical trial services market.
Get Your Free Sample Report Now – Explore Exclusive Market Insights:
https://www.thebusinessresearchcompany.com/sample.aspx?id=15535&type=smp
#What is the Anticipated CAGR of theCell And Gene Therapy Clinical Trial Services Market, and What Factors Will Drive It?
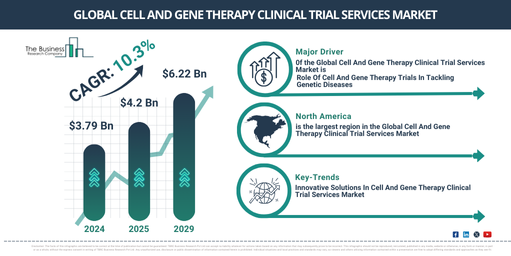
Over the past few years, the market size for clinical trial services for cell and gene therapy has seen a significant increase. The forecast suggests a rise from $3.79 billion in 2024, up to $4.2 billion in 2025, demonstrating a compound annual growth rate (CAGR) of 10.7%. The prior growth can be attributed to factors such as the rising prevalence of long-term illnesses, heightened demand for efficacious treatments for rare genetic disorders, better government funding for regenerative medicine research, improved comprehension of the molecular mechanisms involved in various diseases, and enhanced recognition of personalized medicine’s potential.
Expected to see a fast-paced expansion in the coming years, the market for cell and gene therapy clinical trial services is envisaged to reach a valuation of $6.22 billion in 2029, with a compound annual growth rate (CAGR) of roughly 10.3%. This growth over the predicted period can be ascribed to factors such as the escalating global occurrence of genetic and auto-immune diseases, the rise in public as well as private sector investment in gene therapy research, increased acceptance of new technologies, and a surge in the number of genetic defects. Key trends in the forecast phase encompass the incorporation of AI, the use of machine learning, the application of personalized medicine, technological advancements, and gene therapies.
You can Directly Purchase the Report Here:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=15535
What Key Market Trends and Innovations Are Shaping the Future of theCell And Gene Therapy Clinical Trial Services Industry?
Leading companies in the cell and gene therapy clinical trial services market are turning to sophisticated technology integrated with customer relationship management (CRM) systems in order to enhance their operational efficiency and secure a competitive edge. These CRM systems deployed by cell and gene therapy clinical trial services enhance coordination and streamline communication with trial participants, healthcare providers, and other relevant parties during the clinical trial process. For example, in April 2023, the healthcare firm AmerisourceBergen Corporation, based in the US, unveiled the Cell and Gene Therapy (CGT) Integration Hub. This platform is designed to boost connectivity and streamline procedures throughout the cell and gene therapy treatment pathway. Backed by AmerisourceBergen’s CRM system, the platform aims to simplify the path-to-care by providing doctors and patient services teams with improved oversight of the therapy development and delivery process. The CGT Integration Hub, equipped with features such as expedited benefits investigation, real-time status tracking, and proactive alerts, is designed to simplify care coordination, eliminate hurdles, and enhance the overall experience of patients and providers in the delivery of cell and gene therapies.
Which Companies Are Leading the Charge in Expanding theCell And Gene Therapy Clinical Trial Services Market Growth?
Major companies operating in the cell and gene therapy clinical trial services market are Thermo Fisher Scientific Inc., Sharp Services LLC, IQVIA, Laboratory Corporation of America Holdings, ICON plc, Syneos Health Inc., Catalent Pharma Solutions Inc., Charles River Laboratories International Inc., Parexel International Corporation, PRA Health Sciences Inc., Covance Inc., Medpace Holdings Inc., BioClinica Inc., Precision Medicine Group LLC, Worldwide Clinical Trials LLC, Clinigen Group plc, Evidera Inc., Advarra LLC, Veristat LLC, Clinipace Inc., Celonic AG, Cromsource Inc., Novotech Pty Ltd., MedSource Holdings Inc., Frontage Laboratories Inc.
Order Your Report Now For A Swift Delivery:
How is the Global Cell And Gene Therapy Clinical Trial Services Market Segemented?
The cell and gene therapy clinical trial services market covered in this report is segmented –
1) By Service: Clinical Trial Design And Planning, Supply And Logistic Services, Regulatory Affairs And Compliance, Data Management And Biostatics, Site Management And Monitoring, Other Services
2) By Therapy Type: Gene Therapy, Cell Therapy, Gene Modified Cell Therapy
3) By Indication: Oncology, Hematology, Metabolic Disorders, Infectious Diseases, Neurology, Cardiovascular Diseases, Musculoskeletal Disorders, Other Indications
4) By End-Use: Pharmaceutical And Biotechnology Companies, Contract Research Organizations, Academic And Research Institutes, Other End-Users
Subsegments:
1) By Clinical Trial Design And Planning: Protocol Development, Trial Feasibility Studies, Trial Design Consultation, Patient Recruitment And Enrollment Strategy, Risk-Based Monitoring Plans
2) By Supply And Logistic Services: Clinical Trial Supply Chain Management, Cold Chain Logistics, Packaging And Labeling Services, Transportation And Distribution Of Biological Samples, Customs And Import/Export Services
3) By Regulatory Affairs And Compliance: Regulatory Strategy And Consulting, Regulatory Submission And Documentation, Compliance With GMP (Good Manufacturing Practices), Regulatory Pathway Consultation, Clinical Trial Applications (CTAs)
4) By Data Management And Biostatistics: Clinical Data Management (CDM), Statistical Analysis And Reporting, Clinical Data Monitoring, Data Integration And Validation, Electronic Data Capture (EDC) Solutions
5) By Site Management And Monitoring: Site Selection And Initiation, Clinical Monitoring And Site Visits, Patient Recruitment And Retention, Site Training And Support, Site Performance Monitoring
6) By Other Services: Patient Advocacy And Engagement, Real-World Evidence (RWE) Studies, Post-Trial Services, Medical Writing And Documentation, Clinical Trial Auditing And Inspection
Gain Exclusive Market Insights—Customize Your Research Report Today for Fast Delivery!
https://www.thebusinessresearchcompany.com/customise?id=15535&type=smp
Which Geographics are Influencing the Growth of the Cell And Gene Therapy Clinical Trial Services Market?
North America was the largest region in the cell and gene therapy clinical trial services market in 2023. It is expected to be the fastest-growing region in the forecast period. The regions covered in the cell and gene therapy clinical trial services market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
Browse Through More Reports Similar to the Global Cell And Gene Therapy Clinical Trial Services Market 2025, By The Business Research Company:
Cell Isolation Global Market Report 2024
https://www.thebusinessresearchcompany.com/report/cell-isolation-global-market-report
Cell Therapy Global Market Report 2024
https://www.thebusinessresearchcompany.com/report/cells-therapy-global-market-report
Cell Lysis And Fractionation Global Market Report 2024
https://www.thebusinessresearchcompany.com/report/cell-lysis-and-fractionation-global-market-report
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Contact us at:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at info@tbrc.info
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
Found this article helpful? Share it on: