What Will The Viral Inactivation Market Look Like In 2023?
The Business Research Company’s global market reports are now updated with the latest market sizing information for the year 2023 and forecasted to 2032
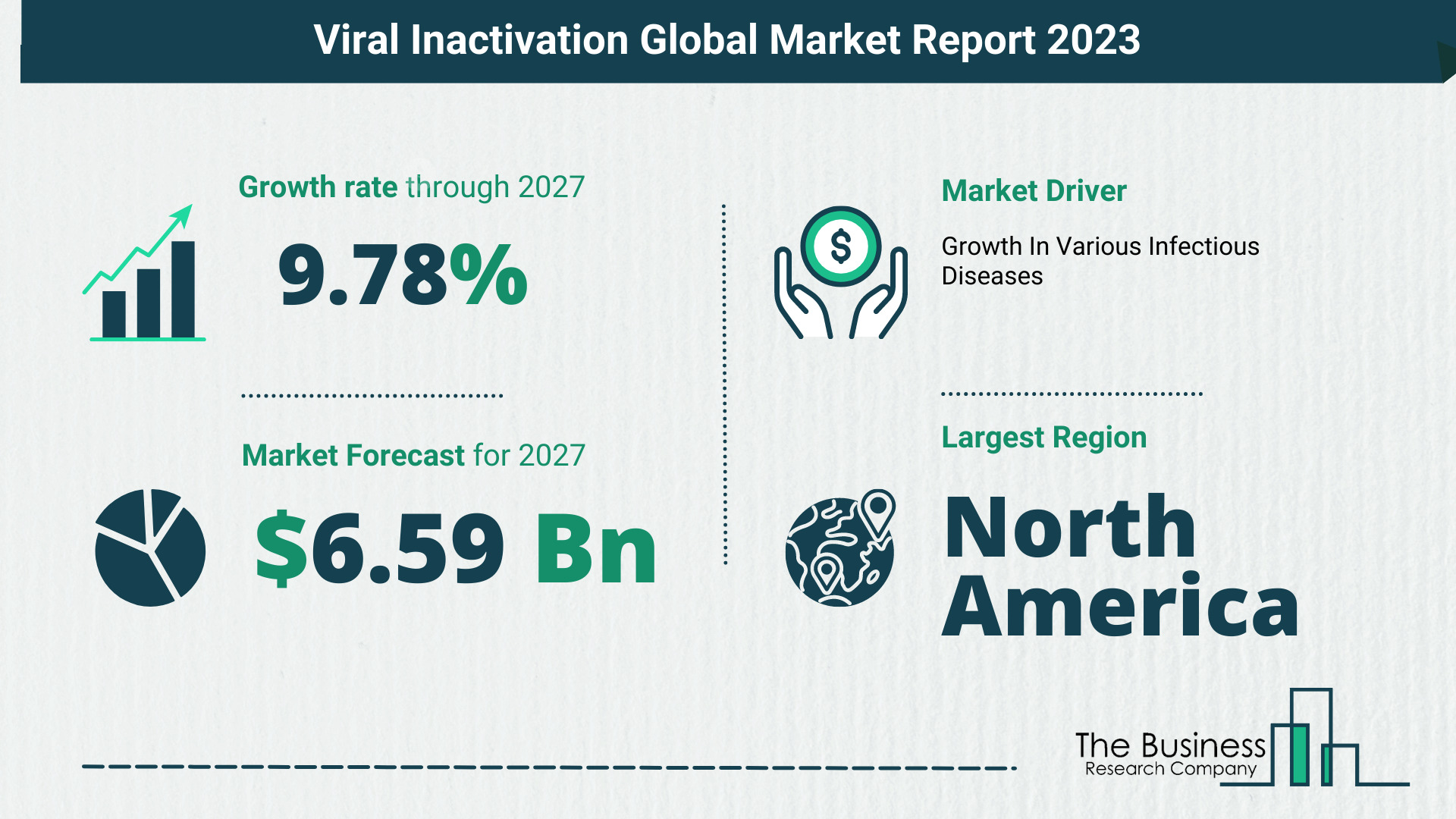
The Business Research Company’s viral inactivation market report forecasts the viral inactivation market size to grow to $6.59 billion by 2027, with a CAGR (compound annual growth rate) of more than 9%.
Learn More On The Viral Inactivation Market Report 2023 – https://www.thebusinessresearchcompany.com/report/viral-inactivation-global-market-report
Viral Inactivation Market Size Forecast
The global viral inactivation market size is expected to grow from $4.15 billion in 2022 to $4.54 billion in 2023 at a compound annual growth rate (CAGR) of 9.35%. The Russia-Ukraine war disrupted the chances of global economic recovery from the COVID-19 pandemic, at least in the short term. The war between these two countries has led to economic sanctions on multiple countries, a surge in commodity prices, and supply chain disruptions, causing inflation across goods and services and affecting many markets across the globe. The global viral inactivation market size is expected to reach $6.59 billion in 2027 at a CAGR of 9.78%.
North America held the largest viral inactivation market share, and Asia-pacific was the fastest-growing region in 2022.
Key Viral Inactivation Market Driver – Growth In Various Infectious Diseases
For instance, in December 2022, according to the ‘Annual Epidemiological Report for 2021’, published by European Centre for Disease Prevention and Control, a Sweden-based, European Government Health Agency, 2021, the distribution of hepatitis C cases and rates per 100,000 population, in Germany, reached 4,718 cases and 5.7 rates respectively from 4,536 cases 5.5 rates in 2020. Therefore, the growing instances of various infectious diseases are driving the growth of the viral inactivation market.
Request for A Sample Of The Global Viral Inactivation Market Report:
https://www.thebusinessresearchcompany.com/sample.aspx?id=10401&type=smp
Key Viral Inactivation Market Trend – Increasing Use Of Technologically Advanced Processes
To maintain their market position, companies in the viral inactivation industry are implementing technologically advanced techniques. WuXi Advanced Therapies, a wholly owned subsidiary of WuXi AppTec, a China-based biopharmaceutical business, for example, launched Tetracycline-Enabled Self-Silencing Adenovirus (TESSATM) Technology in March 2022. By conducting all assay formulation, biosafety, viral clearance, product release, and testing in-house, this cutting-edge technique has enabled the approval of new medicines. This approach is a novel and revolutionary way for manufacturing adeno-associated virus (AAV) at Good Manufacturing Practises (GMP) standards without the requirement for transfection.
Viral Inactivation Market Segment
1) By Product: Kits And Reagents, Systems, Services
2) By Method: Solvent Detergent Method, pH Adjustment Method, Pasteurization, Other Methods
3) By Application: Vaccines And Therapeutics, Tissues And Tissue Products, Blood And Blood Products, Other Applications
4) By End User: Pharmaceutical And Biotechnology Companies, Contract Research Organizations, Blood Banks, Hospital, Academic Research Institutes, Other End Users
Viral Inactivation Market Major Players and Strategies
Major players in the viral inactivation market are Merck & Co., PARKER HANNIFIN CORP, Sartorius AG, Texcell, SGS SA, Charles River Laboratories., Clean cells, Rad Source Technologies, Wuxi Apptec, Sigma-Aldrich Co., Thermo Fisher Scientific Inc., Macopharma, Cerus Corporation., TERUMO BCT INC., Weigao Group, SCI Automation., Pall Corporation And Eurofins Scientific and General Electric Company.
Asahi Kasei Medical, a Japanese healthcare firm specialising in chemical manufacturing, acquired Bionova Scientific for an unknown sum in June 2022. Asahi Kasei receives access to Bionova’s 36,000-square-foot manufacturing facility, which contains two 2,000-litre mammalian cell culture trains and the capability of producing numerous monoclonal antibodies and other recombinant proteins. Following the acquisition, Asahi Kasei intends to capitalise on its existing operations in bioprocess supplies, equipment, and biosafety testing services, as well as Bionova Scientific’s biologics CDMO division. Bionova Scientific is a firm established in the United States that specialises in the cGMP manufacturing of biologics as well as downstream purification technologies for viral inactivation and viral filtering.
The Viral Inactivation Global Market Report 2023 covers regional data on viral inactivation market size, viral inactivation market trends and drivers, opportunities, strategies, and viral inactivation market competitor analysis. The countries covered in the viral inactivation market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, the UK, and the USA, and the major seven regions are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, the Middle East, and Africa.
The process of inactivating viral contamination by exposing the bioprocess fluid to conditions that denature the viral protein while retaining the active ingredient of the product is referred to as viral inactivation. Viral inactivation is most typically utilized in the preparation of blood plasma and monoclonal antibodies.
View More Reports Related To The Viral Inactivation Market –
Viral Vectors And Plasmid DNA Global Market Report 2023
Virus Filtration Global Market Report 2023
Coronavirus (COVID-19) Current Therapy Global Market Report 2023
Contact us at:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at [email protected]
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
Found this article helpful? Share it on: