Congenital Diaphragmatic Hernia Drugs Market Outlook 2025: Mapping Growth, Innovation, and Regional Shifts
Discover trends, market shifts, and competitive outlooks for the congenital diaphragmatic hernia drugs industry through 2025-2034 with The Business Research Company’s reliable data and in-depth research
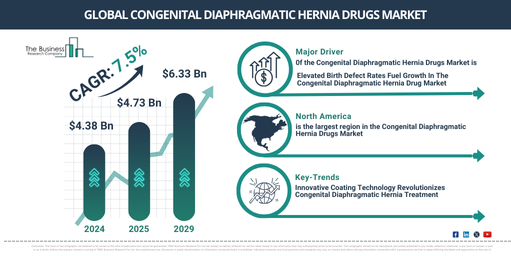
What Is the Current and Projected Market Size of the Congenital Diaphragmatic Hernia Drugs Market Through 2034?
In previous years, there has been significant growth in the size of the market for drugs treating congenital diaphragmatic hernia. The market is set to expand from a value of $4.38 billion in 2024 to $4.73 billion in 2025, representing a compound annual growth rate (CAGR) of 8.1%. Factors such as a high number of congenital diaphragmatic hernia cases, heightened awareness of the condition, and government initiatives have all contributed to the growth of this market during the historic period.
In the coming years, the market for drugs treating congenital diaphragmatic hernia is predicted to experience robust expansion. The market is forecasted to reach $6.33 billion by 2029, registering a compound annual growth rate (CAGR) of 7.5%. The projected growth during this period can be credited to rising healthcare spending, incentives for rare diseases, expanding patient advocacy groups, regenerative medicine, and targeted therapies. Key trends that are expected to shape the market during the forecast period incorporate advancements in medical research, drug development, fetal surgery, pharmacological lung growth, an increasing preference for personalized medicine, the emergence of new and innovative drug delivery systems, and the adoption of digital health technologies.
Download a free sample to assess the report’s scope and structure:
https://www.thebusinessresearchcompany.com/sample.aspx?id=12928&type=smp
What External and Internal Drivers Are Contributing to the Growth of the Congenital Diaphragmatic Hernia Drugs Market?
The congenital diaphragmatic hernia drug market is anticipated to expand due to an increase in the rate of birth defects. Birth defects are functional or structural irregularities existing at birth that have the potential to impact the overall health and growth of the newborn. Treatment strategies for congenital diaphragmatic hernia cover prenatal screening, genetic counseling, and specific therapies aimed at reducing the incidence of birth defects resulting from congenital diaphragmatic hernia. These strategies can advance early intervention, personalized care, and consequently, enhance the quality of life for affected individuals. The World Health Organization (WHO) reported in March 2023 that every year, around 8 million infants are born with birth defects. The majority of these issues, about 90%, occur in low- to middle-income countries. Additionally, it is estimated that congenital disorders contribute to the deaths of approximately 240,000 newborns globally within their first 28 days. Consequently, the increasing rate of birth defects foster the growth of the congenital diaphragmatic hernia drug market. Secondly, the surge in cases of congenital diaphragmatic hernia (CDH) is projected to propel the congenital diaphragmatic hernia drug market. CDH is a birth defect characterized by an abnormal opening in the diaphragm, which separates the chest and abdominal cavity. Medications aimed at treating CDH may enhance patient outcomes, improve lung function and mitigate pulmonary hypertension. The Cleveland Clinic, a renowned US-based academic medical center, revealed in October 2022 that 1 in every 2,500 to 3,500 live births resulted in CDH. Furthermore, survival rates for infants diagnosed with CDH range between 70% and 90%. Thus, the rising occurrence of congenital diaphragmatic hernia (CDH) fuels the congenital diaphragmatic hernia drug market.
What Segment Types Define the Congenital Diaphragmatic Hernia Drugs Market Structure?
The congenital diaphragmatic hernia drugs market covered in this report is segmented –
1) By Type: Posterolateral Bochdalek Hernia, Anterior Morgagni Hernia, Hiatal Hernia
2) By Treatment: Extracorporeal Membrane Oxygenation, Other Treatments
3) By Diagnosis: Prenatal, Postnatal
4) By End-User: Hospitals, Homecare, Specialty Clinics, Other End Users
Subsegments:
1) By Posterolateral Bochdalek Hernia: Surgical Interventions, Medications for Symptoms
2) By Anterior Morgagni Hernia: Surgical Interventions, Medications for Symptoms
3) By Hiatal Hernia: Surgical Interventions, Medications
Request customized data on this market:
https://www.thebusinessresearchcompany.com/customise?id=12928&type=smp
Which Geographic Areas Hold the Strongest Growth Potential in the Congenital Diaphragmatic Hernia Drugs Market?
North America was the largest region in the congenital diaphragmatic hernia drugs market in 2024. The regions covered in congenital diaphragmatic hernia drugs market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa.
What Long-Term Trends Are Transforming the Competitive Landscape of the Congenital Diaphragmatic Hernia Drugs Market?
In an attempt to solidify their standing in the market for congenital diaphragmatic hernia (CDH), leading businesses are bringing forth innovations in treatment approaches using coating technology. This technique entails the application of a distinctive layer or coating onto a synthetic mesh used in hernia repair that includes medication. In April 2022, for example, Ariste Medical, a US-based drug and device firm in the pre-market stage, gained approval from the U.S. Food and Drug Administration (FDA) to sell its drug-embedded synthetic hernia mesh. The mesh, targeted at decreasing the chances of microbial build-up during surgical placement, incorporates the advantages of a polypropylene mesh by means of a biocompatible coating that enables antibiotic residence to suppress bacterial growth. Devices both implantable and non-implantable can benefit from this coating technology, and its prospective use in materials like polyurethane and ePTFE has been demonstrated. Additionally, Ariste Medical’s innovative coating technology could be utilized for ePTFE surgical implants, including those used in hernia repair surgeries.
View the full report here:
What Is the Definition of the Congenital Diaphragmatic Hernia Drugs Market?
Congenital diaphragmatic hernia (CDH) is a rare condition that occurs when there is a hole in the diaphragm, the muscle that separates the chest from the abdomen. Congenital diaphragmatic hernia (CDH) drugs refer to the medications used in the treatment of Congenital diaphragmatic hernia and help reduce the severity of the disease and manage the symptoms and complications associated with the disease.
Purchase the full report and get a swift delivery:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=12928
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Get in touch with us:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at info@tbrc.info
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model