Uterine Fibroid Embolization DeviceMarket Overview 2025–2034: CAGR Trends, Long-Term Growth Paths, and Business Implications
Discover trends, market shifts, and competitive outlooks for the uterine fibroid embolization device industry through 2025-2034 with The Business Research Company’s reliable data and in-depth research
How Has the Uterine Fibroid Embolization Device Market Growth Performance Trended Historically, And What Lies Ahead?
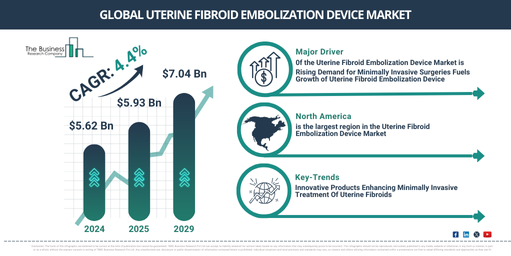
The market size for uterine fibroid embolization devices has shown robust growth in the past few years. It is projected to increase from $5.62 billion in 2024 to $5.93 billion in 2025, exhibiting a compound annual growth rate (CAGR) of 5.6%. The substantial market growth in the prior period can be credited to the surge in the occurrence of uterine fibroids, the preference for non-invasive alternatives, initiatives to raise awareness and education, focus on the health of women, transition to outpatient procedures, and training programs for physicians.
The market for uterine fibroid embolization devices is anticipated to experience consistent expansion in the approaching years, culminating in a value of $7.04 billion by 2029, reflecting a compound annual growth rate (CAGR) of 4.4%. This projected growth can be linked to the rising public awareness about non-invasive treatments, the global proliferation of health care services, increased female health initiatives, integration with multi-disciplinary care, and outpatient service expansion. The period under forecast is likely to be defined by trends such as the heightened implementation of minimally invasive procedures, developments in embolic agents, customized treatment strategies, emphasis on outpatient ufe procedures, the incorporation of imaging technologies, and studies pertaining to bioresorbable embolic agents.
Download a free sample to assess the report’s scope and structure:
https://www.thebusinessresearchcompany.com/sample.aspx?id=13551&type=smp
What Are the Primary Drivers Supporting the Market Growth of the Uterine Fibroid Embolization Device Market?
The surge in minimally invasive surgeries is predicted to bolster the uterine fibroid embolization device market. These type of surgeries involve the use of the natural body openings or small incisions to reach and treat the internal structures, tissues, or organs with the aim to reduce the trauma inflicted on the patient’s body. Uterine fibroid embolization devices are key instruments empowering these minimally invasive surgeries to efficiently cure uterine fibroids. Created with meticulous precision, these devices minimize invasiveness by employing small incisions and minimize disruption to nearby tissues. They help address the growing need for less invasive medical procedures, thus enhancing patient outcomes. For example, The Aesthetic Society, a US-based body dedicated to plastic surgery and cosmetic medicine, stated in August 2023 that aesthetic procedures worth more than $11.8 billion were undertaken by Americans, marking a rise of 2% from the preceding year. As such, the mounting demand for minimally invasive surgeries is fostering the expansion of the uterine fibroid embolization device market. The uterine fibroid embolization devices market is expected to be driven by a growing aging population. The term aging population refers to the ratio of individuals aged 65 and over within a population group. Uterine fibroid embolization devices assist in tackling symptomatic fibroids among the elderly, offering a less invasive solution to surgery, and mitigating symptoms such as excessive bleeding and pelvic discomfort. For example, the Switzerland-based intergovernmental body, World Health Organization, reported in October 2022 that the population segment aged 60 and above had soared to 1.4 billion in 2022. It is predicted that by 2050, the global population of individuals aged 60 and above will double to 2.1 billion. Hence, the swelling aging population is leading to the expansion of the uterine fibroid embolization devices market.
Which Primary Segments of the Uterine Fibroid Embolization Device Market Are Driving Growth and Industry Transformations?
The uterine fibroid embolization device market covered in this report is segmented –
1) By Technology: Surgical Techniques, Laparoscopic Techniques, Ablation Techniques, Embolization Techniques
2) By Mode Of Treatment: Invasive Treatment, Minimally Invasive Treatment, Non-Invasive Treatment
3) By End User: Hospitals, Clinics, Ambulatory Surgical Center
Subsegments:
1) By Surgical Techniques: Open Surgery, Myomectomy
2) By Laparoscopic Techniques: Laparoscopic Myomectomy, Laparoscopic Hysterectomy
3) By Ablation Techniques: Radiofrequency Ablation, Microwave Ablation, Cryoablation
4) By Embolization Techniques: Transcatheter Arterial Embolization (TAE), Uterine Artery Embolization (UAE)
Request customized data on this market:
https://www.thebusinessresearchcompany.com/customise?id=13551&type=smp
Which Regions Are Key Players in the Growth of the Uterine Fibroid Embolization Device Market?
North America was the largest region in the uterine fibroid embolization device market in 2024. The regions covered in the uterine fibroid embolization device market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
What Are the Most Significant Market Trends in the Uterine Fibroid Embolization Device Market?
In the uterine fibroid embolization device market, leading corporations such as Medtronic Private Limited are innovating and crafting products like the TruClear system. They aim to fulfil the increasing demand for less intrusive solutions for uterine fibroids. The TruClear technology enhances the security and effectiveness of uterine fibroid treatments. The system operates mechanically in its function as a hysteroscopic tissue remover for intrauterine abnormalities (IUA). This flexible catheter makes its way into the uterus via the cervix. In November 2022, the India-based industrial medical equipment manufacturer, Medtronic Private Limited, introduced the TruClear system. This system provides a reliable and efficient treatment for IUA. Furthermore, the compact catheter, equipped with a circulating nitinol wire loop, consistent inflow and outflow, incorporated suction, and high-definition imaging, has improved the accuracy and safety of treating uterine fibroids.
View the full report here:
What Parameters Are Used to Define the Uterine Fibroid Embolization Device Market?
A uterine fibroid embolization device is a medical device that is used to treat noncancerous tumors using a minimally invasive process. Uterine fibroids are noncancerous growths of the uterus. This device restricts blood supply to uterine fibroids, causing them to shrink and eventually disappear.
Purchase the full report and get a swift delivery:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=13551
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Get in touch with us:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at info@tbrc.info
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model