Adeno-Associated Virus (AVV) Vectors In Gene Therapy Market in Focus: Forecasting Growth Drivers, Scaling Potential, and Global Opportunities
We’ve updated all our reports with current data on tariff changes, trade developments, and supply chain shifts affecting key industries.
What Are the Key Milestones in the Adeno-Associated Virus (AVV) Vectors In Gene Therapy Market’s Growth Trajectory From 2025 To 2034?
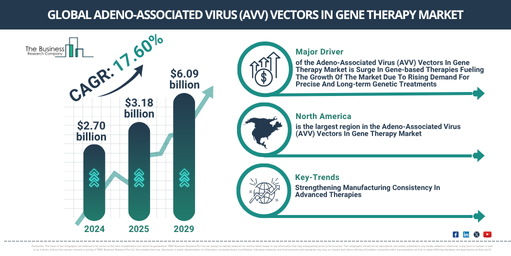
The market size for adeno-associated virus (AVV) vectors in gene therapy has seen a significant expansion in the past few years. The market is projected to balloon from $2.70 billion in 2024 to $3.18 billion in 2025, reflecting a compound annual growth rate (CAGR) of 17.7%. The exponential growth during the historical period is a result of escalated investments in gene therapy research, the surging uptake of gene editing technologies, a growing number of genetic disorders and unmet medical requirements, an increased need for targeted therapies, and an uptick in partnerships between biotech firms and research institutions.
The market size for adeno-associated virus (AVV) vectors utilized in gene therapy is predicted to undergo swift expansion in the following years, reaching a value of $6.09 billion by 2029, with a projected compound annual growth rate (CAGR) of 17.6%. The majority of this growth in the forecast period is expected to come from enhancements in AAV capsid engineering, a heightened emphasis on precision medicine, augmented financial investment in genetic research and biotechnology, an upsurge in gene therapy clinical trials and approvals, and an increased demand for personalized treatments. Key trends during the forecast period include AI advancements applied to AAV capsid engineering, the creation of hybrid AAV vectors capable of holding larger genetic payloads, the innovative approach to bispecific antibody-mediated AAV targeting, advancements in scalable AAV production and purification methodologies, and the creation of versatile AAV platforms capable of handling various viral vectors.
Download a free sample to assess the report’s scope and structure:
https://www.thebusinessresearchcompany.com/sample.aspx?id=24135&type=smp
What Are the Core Market Drivers Propelling Growth in the Adeno-Associated Virus (AVV) Vectors In Gene Therapy Industry?
The growth of the adeno-associated virus (AAV) vectors in the gene therapy market is anticipated to be fueled by the escalating emphasis on gene-based treatments. These treatments manipulate or modify genes to treat, prevent, or cure diseases at a molecular level. The burgeoning focus on gene-based therapies is driven by advancements in genetic research which heightens the accuracy and potency of gene editing methodologies. The utilization of adeno-associated virus (AAV) vectors in gene therapy facilitates the development of gene-based therapies by safely and efficiently introducing therapeutic genes into target cells, due to their minimal immunogenicity and capacity for long-term gene expression. For example, data from IQVIA, a US-based firm providing advanced analytics and technological solutions for the life sciences industry, showed that global expenditure on cell and gene therapies hit $5.9 billion in 2023, a 38% rise from 2022. Consequently, the growing emphasis on the development of gene-based therapies is fueling the expansion of the adeno-associated virus (AAV) vectors market.
How Is the Adeno-Associated Virus (AVV) Vectors In Gene Therapy Market Segmented?
The adeno-associated virus (AVV) vectors in gene therapy market covered in this report is segmented –
1) By Type of Therapy: Gene Augmentation, Immunotherapy, Other Type of Therapy
2) By Type of Gene Delivery Method Used: Ex Vivo, In Vivo
3) By Scale of Operation: Preclinical, Clinical, Commercial
4) By Target Therapeutic Area: Genetic Disorders, Hematological Disorders, Infectious Diseases, Metabolic Disorders, Ophthalmic Disorders, Muscle Disorders, Neurological Disorders, Other Target Therapeutic Area
Subsegments:
1) By Gene Augmentation: Monogenic Disorders, Neurological Disorders, Muscular Disorders, Ophthalmological Disorders, Metabolic Disorders
2) By Immunotherapy: Oncology, Infectious Diseases, Autoimmune Disorders, Vaccine Development, T-Cell Engineering
3) By Other Type of Therapy: Gene Editing Support, RNA Interference, Neuroprotection and Neuroregeneration, Anti-Inflammatory Applications, Regenerative Medicine
Request customized data on this market:
https://www.thebusinessresearchcompany.com/sample.aspx?id=24135&type=smp
Which Regions Are Driving the Next Phase of the Adeno-Associated Virus (AVV) Vectors In Gene Therapy Market Growth?
North America was the largest region in the adeno-associated virus (AVV) vectors in gene therapy market in 2024. The regions covered in the adeno-associated virus (AVV) vectors in gene therapy market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
What Key Market Trends and Innovations Are Shaping the Future of the Adeno-Associated Virus (AVV) Vectors In Gene Therapy Industry?
Prominent companies in the adeno-associated virus (AAV) vector segment of the gene therapy market are honing their focus on sophisticated innovation, including the adaptation of vector diversity for assay versatility. This is expected to augment the specificity of target tissue, enhance therapeutic effectiveness and speed up the formulation of customized gene therapies for an array of medical conditions. The modified vector diversity for assay versatility involves the use of numerous AAV serotypes or constructs, aiding a range of analytical examinations and therapeutic uses. To illustrate, Charles River Laboratories, a pharmaceutical firm in the US, launched new reference materials for adeno-associated virus (AAV) and lentiviral vectors (LVV) in May 2024. These resources are designed to meet the increasing demands of cell and gene therapy (CGT) advancement. The portfolio eases the transition from early-stage research to GMP-grade production, providing a more streamlined process and augmenting the consistency in viral vector manufacturing. This launch aims to tackle a significant hurdle in elevating CGT programs to clinical and commercial readiness.
View the full report here:
How Is the Adeno-Associated Virus (AVV) Vectors In Gene Therapy Market Defined and What Are Its Core Parameters?
Adeno-associated virus (AAV) vectors in gene therapy are advanced delivery tools that transport genetic material into cells to treat various genetic disorders. Their primary goal is to enable targeted, long-lasting therapeutic effects by correcting or modifying faulty genes. AAV vectors are valued for their safety, low immunogenicity, and ability to deliver genes to both dividing and non-dividing cells, supporting the development of precise and durable gene therapies that advance personalized medicine and transform the treatment of rare and inherited diseases.
Purchase the full report and get a swift delivery:
https://www.thebusinessresearchcompany.com/customise?id=24135&type=smp
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Get in touch with us:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at info@tbrc.info
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model