Endotoxin Testing Market 2025-2034: Key Highlights, Growth Dynamics, and Emerging Trends
Discover trends, market shifts, and competitive outlooks for the endotoxin testing industry through 2025-2034 with The Business Research Company’s reliable data and in-depth research
#What Is the Estimated Market Size of the Endotoxin Testing Market In 2029?#_x000D_
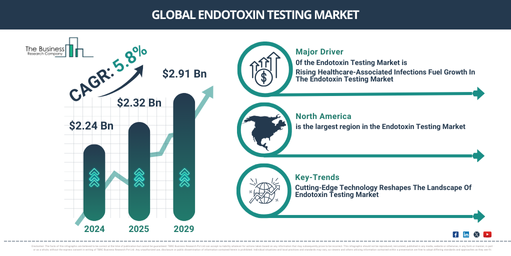
The scale of the endotoxin testing market has been progressively expanding over the past few years. The market, which stood at $2.24 billion in 2024, is projected to increase to $2.32 billion in 2025, marking a compound annual growth rate (CAGR) of 3.6%. The expansion during the historic period was driven by the escalating demand for biologics and biosimilars, heightened awareness of endotoxin testing significance, and growing incidence of infectious diseases._x000D_
_x000D_
In the coming years, we can expect a significant expansion in the endotoxin testing market. The projected market size is set to reach $2.91 billion by 2029, with an annual compound growth rate of 5.8%. The projected growth within this period can be credited to the enlargement of the biopharmaceutical sector, amplified expenditure in endotoxin testing research and development, and the expansion of global healthcare. Key trends anticipated during this forecast period are the introduction of inventive endotoxin detection tools, the prevalence of endotoxin testing services, and a surge in the demand for these services._x000D_
_x000D_
#Download a free sample to assess the report’s scope and structure:#_x000D_
https://www.thebusinessresearchcompany.com/sample.aspx?id=12932&type=smp_x000D_
_x000D_
#Which Primay Drivers Are Accelerating Growth in the Endotoxin Testing Market?#_x000D_
The escalating incidence of infections related to healthcare is predicted to stimulate the expansion of the endotoxin testing market in the future. Referring to infections obtained during medical treatment that were initially absent upon hospital admission, healthcare-associated infections usually result from infected medical devices or procedures. This situation necessitates stringent testing to safeguard patient health. Endotoxin testing services considerably inhibit healthcare-associated infections by evaluating bacterial endotoxin levels in medical apparatus, particularly injectable pharmaceutical products and implantable medical devices. For instance, pneumonia was reported by the United Nations International Children’s Emergency Fund (UNICEF), a US branch of the United Nations, as the highest child-killing infectious disease in December 2022, claiming over 700,000 child lives annually, or roughly 2,000 per day. Additionally, according to the World Health Organization’s May 2022 report, a Swiss agency of the United Nations, healthcare-associated sepsis affects 24% of patients annually, and 52.3% of these patients receiving treatment in an intensive care unit succumb each year. Hence, the escalating incidence of healthcare-associated infections stimulates the expansion of the endotoxin testing market. The endotoxin testing market’s growth is anticipated to be fueled by a rising demand for biologics for various tests. Biologics, also recognized as biological products or biopharmaceuticals, include pharmaceuticals produced utilizing living beings, cells, or biological processes. It’s crucial to perform endotoxin testing for biologics to ensure they meet safety, quality, and regulatory compliance. For instance, the European Pharmaceutical Review (EPR), a UK-based NGO, indicated in May 2022 that in the forthcoming five years, biologics are likely to vastly eclipse innovative small molecule sales, projecting a $120 billion sales increase by 2027. This signals a major transition in the pharmaceutical market towards biological goods. Therefore, the rising demand for biologics to undertake various tests propels the expansion of the endotoxin testing market._x000D_
_x000D_
#Which Primary Segments of the Endotoxin Testing Market Are Driving Growth and Industry Transformations?#_x000D_
The endotoxin testing market covered in this report is segmented –_x000D_
_x000D_
1) By Test Type: LAL (Limulus Amebocyte Lysate) Test, Chromogenic Tests, Turbidimetric Tests, Gel Clot Tests, MAT Test, Rabbit Pyrogen Test, Recombinant Factor C (rFC) Assay_x000D_
2) By Application: Medical Devices, Pharmaceuticals, Packaging, Raw Materials_x000D_
3) By End-User: Hospitals, Laboratories, Research Institutes_x000D_
_x000D_
Subsegments:_x000D_
1) By LAL (Limulus Amebocyte Lysate) Test: Gel Clot LAL Test, Kinetic LAL Test, Endpoint LAL Test_x000D_
2) By Chromogenic Tests: Chromogenic LAL Test, Chromogenic Factor C Assay_x000D_
3) By Turbidimetric Tests: Turbidimetric LAL Test, Turbidimetric Factor C Assay_x000D_
4) By Gel Clot Tests: Gel Clot LAL Test_x000D_
5) By MAT Test (Monocyte Activation Test): Direct MAT, Indirect MAT_x000D_
6) By Rabbit Pyrogen Test: Standard Rabbit Pyrogen Test_x000D_
7) By Recombinant Factor C (rFC) Assay: RFC Assay For Endotoxin Detection_x000D_
_x000D_
#Request customized data on this market:#_x000D_
https://www.thebusinessresearchcompany.com/customise?id=12932&type=smp_x000D_
_x000D_
#Which Regions Are Key Players in the Growth of the Endotoxin Testing Market?#_x000D_
North America was the largest region in the endotoxin testing market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in endotoxin testing market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa._x000D_
_x000D_
#Which Technological Trends Are Reshaping the Endotoxin Testing Industry Dynamics?#_x000D_
Leading corporations in the endotoxin testing market are concentrating on integrating new technological advancements to maintain their market dominance. For example, Lonza Group AG, a pharmaceutical, biotechnology, and nutrition production firm based in Switzerland, unveiled the Nebula absorbance reader, a novel absorbance microplate reader for simplified endotoxin and pyrogen testing in August 2023. This device is entirely integrated with the latest WinKQCL software provided by Lonza, assisting with data integrity compliance, accelerating training and diminishing validation workload. There’s no longer a need for WinKQCL software users to familiarize with new software in order to operate the modern reader, which ultimately minimizes training requirements. The Nebula Absorbance Reader serves as a cutting-edge and technologically superior reader that delivers results similar to those commonly obtained with the ELx808, an industry-standard absorbance reader that Lorenz has discontinued selling. It is also crafted to be compatible with and fulfill the stringent expectations of Lonza’s absorbance-based endotoxin assays, like the Lonza PYROGENT 5000 Turbidimetric and Kinetic-QCL Chromogenic Endotoxin Assays._x000D_
_x000D_
#View the full report here:#_x000D_
https://www.thebusinessresearchcompany.com/report/endotoxin-testing-global-market-report_x000D_
_x000D_
#What Parameters Are Used to Define the Endotoxin Testing Market?#_x000D_
Endotoxin testing, or the bacterial endotoxins test (BET), refers to an in vitro assay that involves assessing bacterial endotoxin levels in substances, particularly pharmaceuticals and medical devices, to ensure product safety and compliance with quality standards. This testing helps prevent potential adverse effects on patients and users._x000D_
_x000D_
#Purchase the full report and get a swift delivery:#_x000D_
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=12932_x000D_
_x000D_
#About The Business Research Company:#_x000D_
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game._x000D_
_x000D_
#Get in touch with us:#_x000D_
The Business Research Company: https://www.thebusinessresearchcompany.com/_x000D_
Americas +1 3156230293_x000D_
Asia +44 2071930708_x000D_
Europe +44 2071930708_x000D_
Email us at info@tbrc.info_x000D_
_x000D_
#Follow us on:#_x000D_
_x000D_
LinkedIn: https://in.linkedin.com/company/the-business-research-company_x000D_
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ_x000D_
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model