Emerging Trends Redefining the Pharmacogenetic Testing Market Landscape: Cloud-Based Data Lake Platforms Revolutionizing Pharmacovigilance And Drug Safety Software Market
Discover trends, market shifts, and competitive outlooks for the pharmacovigilance and drug safety software industry through 2025-2034 with The Business Research Company’s reliable data and in-depth research
#What Are the Projected Market Size and Growth Rates for the Pharmacovigilance And Drug Safety Software Market From 2025 To 2029?#_x000D_
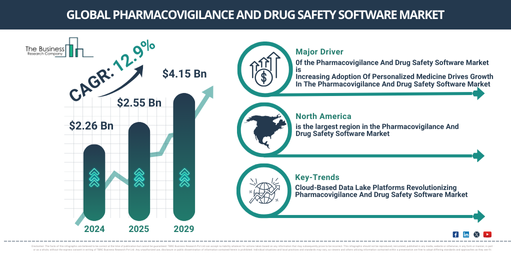
In recent times, the market size of pharmacovigilance and drug safety software has experienced accelerated growth. The market, slated to grow from $2.26 billion in 2024 to $2.55 billion in 2025, forsights a compound annual growth rate (CAGR) of 13.2%. The remarkable growth observed in the historical period is a result of the increasing demand for pharmacovigilance and drug safety software._x000D_
_x000D_
Expectations are high for the pharmacovigilance and drug safety software market, as it’s predicted to experience significant expansion in the coming years. It is anticipated to escalate to $4.15 billion by 2029, boasting a compound annual growth rate (CAGR) of 12.9%. The expansion during the forecast period can be credited to an upsurge in adverse drug reaction incidents and the worldwide spread of pharmacovigilance, escalating incidences of adverse drug reactions, growing intricacy in drug safety regulations, enhancement in the creation of new drugs and therapies, heightening prevalence of adverse drug reactions (ADRs), and a burgeoning number of adverse drug reactions. Trends to look out for during the forecast period encompass automation and AI assimilation, cloud-based strategies, technological inventiveness, progression in technologies, and a rise in healthcare digitalization._x000D_
_x000D_
#Download a free sample to assess the report’s scope and structure:#_x000D_
https://www.thebusinessresearchcompany.com/sample.aspx?id=19140&type=smp_x000D_
_x000D_
#What Are the Core Market Drivers Propelling Growth in the Pharmacovigilance And Drug Safety Software Industry?#_x000D_
Continued interest in personalized medicine, which customizes healthcare treatments based on individual patient characteristics, is expected to drive the pharmacovigilance and drug safety software market. This surge in personalized medicine can be attributed to several factors including improved results from treatments, emphasis on early detection and prevention, and cost saving benefits. The successful implementation of personalized medicine relies heavily on pharmacovigilance and drug safety software. By using pharmacogenetics, real-time data evaluation, post-marketing watchfulness, and customized medication reports, these tools greatly increase the efficacy and safety of personalized therapies. They also aid in implementing strong risk management strategies and address ethical considerations. As an example, the FDA gave approval to 16 new personalized treatments for rare diseases patients in 2023, a noticeable increase from 6 in 2022, as reported in February 2024 by the US based non-profit organization, the Personalized Medicine Coalition. Thus, the rising preference for personalized medicine can be linked to the growth of the pharmacovigilance and drug safety software market._x000D_
_x000D_
#How Is the Pharmacovigilance And Drug Safety Software Market Segmented?#_x000D_
The pharmacovigilance and drug safety softwaremarket covered in this report is segmented –_x000D_
_x000D_
1) By Software Type: Adverse Event Reporting Software, Drug Safety Audits Software, Issue Tracking Software, Fully Integrated Software_x000D_
2) By Delivery Mode: On-premise, Cloud-based_x000D_
3) By End User: Pharmaceutical And Biotechnology Companies, Contract Research Organizations, Business Process Outsourcing Firms, Other End Users_x000D_
_x000D_
Subsegments:_x000D_
1) By Adverse Event Reporting Software: Spontaneous Reporting Systems, EHR Integration Solutions, Mobile Reporting Applications_x000D_
2) By Drug Safety Audits Software: Audit Management Solutions, Compliance Tracking Tools, Reporting And Analytics Modules_x000D_
3) By Issue Tracking Software: Incident Reporting Systems, Workflow Management Tools, Collaboration Platforms_x000D_
4) By Fully Integrated Software: Pharmacovigilance Platforms, Regulatory Compliance Systems, Data Analytics And Visualization Tools_x000D_
_x000D_
#Request customized data on this market:#_x000D_
https://www.thebusinessresearchcompany.com/customise?id=19140&type=smp_x000D_
_x000D_
#Which Regions Are Driving the Next Phase of the Pharmacovigilance And Drug Safety Software Market Growth?#_x000D_
North America was the largest region in the pharmacovigilance and drug safety software market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the pharmacovigilance and drug safety software market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa._x000D_
_x000D_
#What Long-Term Trends Are Transforming the Competitive Landscape of the Pharmacovigilance And Drug Safety Software Market?#_x000D_
Key players in the pharmacovigilance and drug safety software market are advancing their technologies, including cloud-based data lake platforms, to bolster patient safety, more efficiently track adverse events, and better ensure regulatory compliance within clinical trials and medical device surveillance after market release. This type of platform is a scalable, centralised storage system that allows enterprises to store, control, and assess vast quantities of both structured and unstructured data. It provides instantaneous data access and can handle advanced analytics, as well as AI/ML tasks. As an example, Thermo Fisher Scientific Inc., a biotech company from the United States, introduced CorEvidence in December 2023 – a groundbreaking proprietary cloud-based data lake platform created to augment pharmacovigilance protocols in clinical research registers. The goal of the platform is to enhance case handling and safety data management, particularly in the realm of post-approval safety investigations._x000D_
_x000D_
#View the full report here:#_x000D_
_x000D_
#How Is the Pharmacovigilance And Drug Safety Software Market Defined and What Are Its Core Parameters?#_x000D_
Pharmacovigilance and drug safety software refers to a suite of digital tools designed to monitor, analyze, and report adverse drug reactions (ADRs) and other safety-related information associated with pharmaceuticals and medical products. This software plays a critical role in ensuring patient safety and regulatory compliance throughout the lifecycle of a drug, from development to post-marketing surveillance._x000D_
_x000D_
#Purchase the full report and get a swift delivery:#_x000D_
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=19140_x000D_
_x000D_
#About The Business Research Company:#_x000D_
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game._x000D_
_x000D_
#Get in touch with us:#_x000D_
The Business Research Company: https://www.thebusinessresearchcompany.com/_x000D_
Americas +1 3156230293_x000D_
Asia +44 2071930708_x000D_
Europe +44 2071930708_x000D_
Email us at info@tbrc.info_x000D_
_x000D_
#Follow us on:#_x000D_
_x000D_
LinkedIn: https://in.linkedin.com/company/the-business-research-company_x000D_
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ_x000D_
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model