What Will The Adalimumab, Infliximab And Etanercept Biosimilars Market Look Like In 2023?
The Business Research Company’s global market reports are now updated with the latest market sizing information for the year 2023 and forecasted to 2032
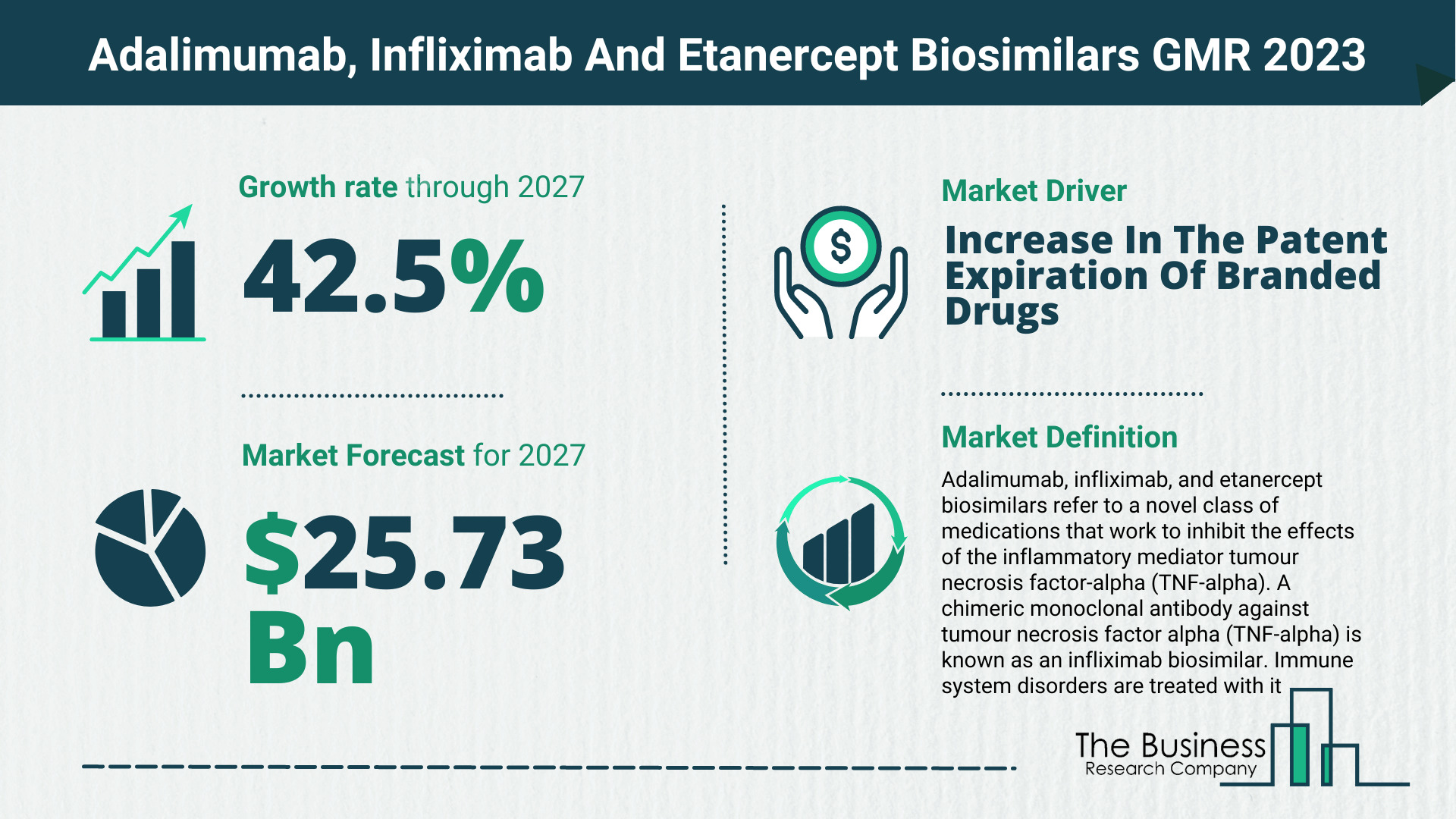
The Business Research Company’s adalimumab, infliximab and etanercept biosimilars market report forecasts the adalimumab, infliximab and etanercept biosimilars market size to grow to $25.73 Billion by 2027, with a CAGR (compound annual growth rate) of more than 42%.
Learn More On The Adalimumab, Infliximab And Etanercept Biosimilars Market Report 2023 – https://www.thebusinessresearchcompany.com/report/adalimumab-infliximab-and-etanercept-biosimilar-global-market-report
Adalimumab, Infliximab And Etanercept Biosimilars Market Size Forecast
The global adalimumab, infliximab and etanercept biosimilars market is expected to grow from $4.76 billion in 2022 to $6.24 billion in 2023 at a compound annual growth rate (CAGR) of 31.1%. The Russia-Ukraine war disrupted the chances of global economic recovery from the COVID-19 pandemic, at least in the short term. The war between these two countries has led to economic sanctions on multiple countries, a surge in commodity prices, and supply chain disruptions, causing inflation across goods and services and affecting many markets across the globe. The adalimumab, infliximab and etanercept biosimilars market is expected to grow from $25.73 billion in 2027 at a CAGR of 42.5%.
Key Adalimumab, Infliximab And Etanercept Biosimilars Market Driver – Increase In The Patent Expiration Of Branded Drugs
For instance, in the United States, the FDA approved inflectra, developed by Hospira (a Pfizer Inc. company), for the treatment of various autoimmune diseases such as rheumatoid arthritis, adult ulcerative colitis, and plaque psoriasis, after the patent expiry of the branded drug Remicade. Similarly, Amgen’s Enbrel had its patent expire in the EU, and with the expiry of the patent, Benepali (a biosimilar of Enbrel) was approved by the European Commission. Humira’s patent expired, and its biosimilars were made available on the market. The FDA has recently (July 2020) approved Hulio, the sixth biosimilar of Humira. Therefore, the patent expiry of branded biologic drugs such as Humira, Enbrel, and Remicade will drive the biosimilar market.
Request for A Sample Of The Global Adalimumab, Infliximab And Etanercept Biosimilars Market Report:
https://www.thebusinessresearchcompany.com/sample.aspx?id=3473&type=smp
Adalimumab, Infliximab And Etanercept Biosimilars Market Segment
1) By Product: Adalimumab Biosimilars (Exemptia, Mabura, Hyrimoz, Hadlima, Abrilada, Others), Infliximab Biosimilars (Inflectra, Renflexis, Ixifi, Avsola), Cipleumab (Erelzi, Eticovo)
2) By Application: Crohn’S Disease, Psoriatic Arthritis, Rheumatoid Arthritis, Ulcerative Colitis, Ankylosing Spondylitis, Plaque Psoriasis, Other Applications
3) By Distribution Channel: Hospital Pharmacies, Retail Pharmacies, Online Pharmacies
Adalimumab, Infliximab And Etanercept Biosimilars Market Major Players and Strategies
Major players in the adalimumab, infliximab and etanercept biosimilars market are Zydus Cadila, Sandoz (Novartis), Samsung Bioepis, AbbVie, Amgen, Boehringer Ingelheim, Pfizer, Celltrion, Mylan, Hetero Drugs Ltd., and Glenmark Pharmaceuticals.
In February 2022, Biocon Limited, an India, based biopharmaceutical company specialising in the development of biological drugs, acquired the biosimilar assets of Viatris, Inc., for an amount of $3.34 billion. Through this acquisition, Biocon establishes itself as a distinctive, vertically integrated worldwide biosimilars leader and accelerates the commercialization of its portfolio of current and future biosimilars directly and its product reach around the world. Viatris, Inc., a US-based biopharmaceutical and healthcare company specialising in the development and specialisation of biological and therapeutic agents.
The Adalimumab, Infliximab And Etanercept Biosimilars Global Market Report 2023 covers regional data on adalimumab, infliximab and etanercept biosimilars market size, adalimumab, infliximab and etanercept biosimilars market trends and drivers, opportunities, strategies, and adalimumab, infliximab and etanercept biosimilars market competitor analysis. The countries covered in the adalimumab, infliximab and etanercept biosimilars market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, the UK, and the USA, and the major seven regions are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, the Middle East, and Africa.
Adalimumab, infliximab, and etanercept biosimilars refer to a novel class of medications that work to inhibit the effects of the inflammatory mediator tumour necrosis factor-alpha (TNF-alpha). A chimeric monoclonal antibody against tumour necrosis factor alpha (TNF-alpha) is known as an infliximab biosimilar. Immune system disorders are treated with it.
View More Reports Related To The Adalimumab, Infliximab And Etanercept Biosimilars Market –
Rituximab Biosimilars Global Market Report 2023
Trastuzumab Biosimilars Global Market Report 2023
Biosimilars Global Market Report 2023
Email us at [email protected]
Call us at:
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Follow us on:
LinkedIn: https://bit.ly/3WzV8lZ
YouTube: https://bit.ly/3jiemhz
Global Market Model: https://bit.ly/3Was30B