Future Outlook of the Adeno-Associated Viral Vectors Market: Growth, Trends, and Emerging Opportunities Explored
Updated 2025 Market Reports Released: Trends, Forecasts to 2034 – Early Purchase Your Competitive Edge Today!
What is the Expected Growth Rate of the Adeno-Associated Viral Vectors Market Based on Industry Projections?
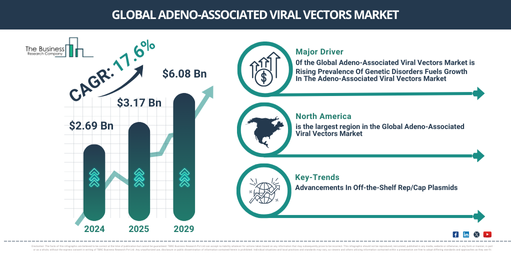
The market size for adeno-associated viral vectors has seen extensive growth in recent years. The market, valued at $2.69 billion in 2024, is predicted to expand to $3.17 billion in 2025 with a compound annual growth rate (CAGR) of 17.9%. Factors contributing to historical growth include vaccine development, increased capital investment, partnerships between academia and industry, the proliferation of clinical trials, as well as raised public consciousness and advocacy.
The market for adeno-associated viral vectors is predicted to undergo swift expansion in the forthcoming years. It is projected to escalate to $6.08 billion by 2029, with a compound annual growth rate (CAGR) of 17.6%. The predicted rise during the forecast timeframe could be due to factors such as the widening scope of therapeutic applications, a higher occurrence rate of genetic and neurological disorders, regulatory endorsement and aid, and the slashing down of production costs. The major trends expected to shape this market in the forecast period are progress in gene therapy, technological breakthroughs, the ability to scale manufacturing processes, partnerships and financial investments, as well as advancements in technology.
What Are the Primary Drivers Supporting the Growth of the Adeno-Associated Viral Vectors Market?
The growth of the adeno-associated viral vectors market is expected to be fueled by the rising incidence of genetic disorders. Genetic disorders, conditions that result from DNA abnormalities leading to physical or developmental anomalies, are on the rise due to improved diagnostic methods, increased awareness, older reproductive age, along with environmental factors and genetic drift. Adeno-associated viral vectors play a crucial role in gene therapy for these genetic disorders by delivering the necessary genes into target cells, potentially treating conditions such as muscular dystrophy or cystic fibrosis. For example, the World Health Organization (WHO), the United Nations’ Switzerland-based agency responsible for global public health, reported in February 2023 that congenital diseases cause approximately 240,000 infant deaths worldwide within 28 days of birth each year. There is an additional death toll of 170,000 children aged between 1 month and 5 years from congenital diseases. Hence, the rising prevalence of genetic disorders is fueling the growth of the adeno-associated viral vectors market.
Explore Comprehensive Insights Into The Global Adeno-Associated Viral Vectors Market With A Free Sample Report:
https://www.thebusinessresearchcompany.com/sample.aspx?id=15969&type=smp
What Companies Are At the Forefront of Innovation in the Adeno-Associated Viral Vectors Market?
Major companies operating in the adeno-associated viral vectors market are:
• Pfizer Inc.
• Astellas Pharma
• Biogen Inc.
• Charles River Laboratories International Inc.
• BioMarin Pharmaceutical Inc.
How Are Emerging Trends in Consumer Behavior Affecting the Adeno-Associated Viral Vectors Market?
In order to gain a competitive advantage in the adeno-associated viral vectors market, key players are striving to offer instant availability of replication-capsid plasmid. This product is frequently used in gene therapy for the production of the adeno-associated virus (AAV) vector and can be obtained from a variety of commercial sources that serve the needs of molecular biology research. For example, in January 2024, Charles River Laboratories International Inc., a pharmaceutical firm based in the United States, launched a range of ready-to-use replication-capsid plasmids that simplify AAV-based gene therapy efforts. This new addition to their product line not only complements their existing lentiviral packaging and AAV Helper plasmid offerings but also reduces manufacturing efforts by up to 66%. These plasmids are produced in batches, following rigorous documentation processes, conforming to CMC guidelines, and issued with a Certification of Analysis (COA) to aid IND and Clinical Trial Application (CTA) submissions.
Secure Your Global Adeno-Associated Viral Vectors Market Report Now for Fast and Efficient Delivery!
Which Key Segments Define the Structure of the Adeno-Associated Viral Vectors Market and Their Growth Potential?
The adeno-associated viral vectors market covered in this report is segmented –
1) By Type Of Therapy: Gene Augmentation, Immunotherapy, Other Type Of Therapies
2) By Type Of Gene Delivery Method Used: Ex Vivo, In Vivo
3) By Target Therapeutic Area: Genetic Disorders, Hematological Disorders, Infectious Diseases, Metabolic Disorders, Ophthalmic Disorders, Muscle Disorders, Neurological Disorders, Other Target Therapeutic Areas
4) By Scale Of Operation: Preclinical, Clinical, Commercial
5) By Application Area: Gene Therapy, Cell Therapy, Vaccines
Subsegments:
1) By Gene Augmentation: Inherited Genetic Disorders, Muscular Dystrophy, Cystic Fibrosis, Hemophilia
2) By Immunotherapy: Cancer Immunotherapy, Viral Infections Immunotherapy, Autoimmune Diseases Immunotherapy
3) By Other Types Of Therapies: Gene Editing, RNA Therapy, Antiviral Therapy
What Regions Are Propelling Growth in the Adeno-Associated Viral Vectors Industry?
North America was the largest region in the adeno-associated viral vectors market in 2023. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the adeno-associated viral vectors market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
What Does the Definition of the Adeno-Associated Viral Vectors Market Include?
Adeno-associated viral vectors (AAV) are small, non-pathogenic viruses commonly used in gene therapy. They are engineered to deliver therapeutic genes into target cells, offering potential treatments for genetic disorders, cancers, and other diseases. AAV’s safety profile and ability to efficiently infect both dividing and non-dividing cells make them valuable tools in biomedical research and clinical applications.
Browse Through More Similar Reports By The Business Research Company:
The Antiviral & Antimicrobial Coatings Global Market Report 2024
Antiviral Combination Therapy Global Market Report 2024
https://www.thebusinessresearchcompany.com/report/antiviral-combination-therapy-global-market-report
Viral Vectors And Plasmid DNA Global Market Report 2024
https://www.thebusinessresearchcompany.com/report/viral-vectors-and-plasmid-dna-global-market-report
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Contact us at:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at info@tbrc.info
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
Found this article helpful? Share it on: