Key Highlights of the Arteriotomy Closure Devices Market 2025-2034: Growth Dynamics, Trends, and Opportunities
Updated 2025 Market Reports Released: Trends, Forecasts to 2034 – Early Purchase Your Competitive Edge Today!
What is the Forecasted Expansion Rate of the Arteriotomy Closure Devices Market Over the 2025–2034 Period?
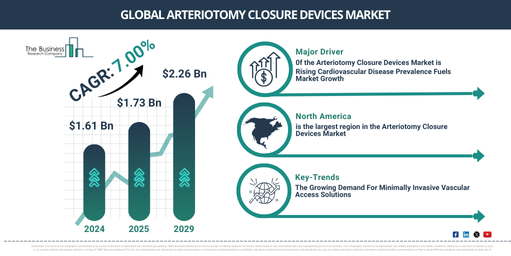
The market for arteriotomy closure devices has seen substantial growth in recent years. It is projected to expand from $1.61 billion in 2024 to $1.73 billion in 2025, with a compound annual growth rate (CAGR) of 7.3%. The escalation in the earlier period is due to a rise in minimally invasive surgeries, an increase in cardiovascular diseases, growing usage of catheterization procedures, a surge in the aging population, and a need for faster recovery times.
Over the coming years, robust expansion is anticipated for the arteriotomy closure devices market. This market is projected to reach $2.26 billion by 2029, with a compound annual growth rate (CAGR) of 7.0%. Factors fueling this growth in the predicted period include a surge in the occurrence of atherosclerosis, an escalation in the cases of coronary artery diseases, increased emphasis on securing patient safety and comfort, the development of health care infrastructure in developing economies, and an increasing need for ambulatory surgical centers. Potential trends for this forecast period encompass minimally invasive methods, the use of bioresorbable materials, automation inclusion in closure processes, enhancing procedural efficiency, and groundbreaking closure devices for large arterial punctures.
Which Primary Drivers Are Supporting the Continued Expansion of the Arteriotomy Closure Devices Market?
The arteriotomy closure devices market is poised for growth, largely due to a surge in cardiovascular diseases (CVDs). CVDs encompass a range of disorders impacting the heart and blood vessels. Rising unhealthful diets, lethargy, obesity, as well as an aging populous, are primarily prompting this increase in CVDs. Arteriotomy closure devices prove critical in cardiovascular interventions by swiftly and safely sealing the punctures in arteries, hence enhancing recovery post procedures like catheterizations and angioplasties, and lowering related complications. The British Heart Foundation, a UK-centered heart research charity, reported in September 2024 that roughly 7.6 million people in the UK, including 4 million men and 3.6 million women, were presently suffering from heart- and circulatory-related diseases. This number could grow by a million by 2030, and potentially swell by another 2 million by 2040, over current data. Therefore, the escalating prevalence of cardiovascular diseases (CVDs) fuels the expansion of the arteriotomy closure devices market.
Explore Comprehensive Insights Into The Global Arteriotomy Closure Devices Market With A Free Sample Report:
https://www.thebusinessresearchcompany.com/sample.aspx?id=20712&type=smp
Who Are the Leading Organizations Fueling the Expansion of the Arteriotomy Closure Devices Market?
Major companies operating in the arteriotomy closure devices market are:
• Cardinal Health Inc._x000D_
• Johnson & Johnson_x000D_
• Abbott Laboratories_x000D_
• Medtronic PLC_x000D_
• Terumo Corporation_x000D_
Which Cutting-Edge Trends Are Expected to Drive the Arteriotomy Closure Devices Market’s Growth?
Firms at the forefront of the arteriotomy closure devices market, such as Haemonetics Corp, are focusing on producing innovative products to boost procedure efficacy, better patient results, and satisfy the increasing need for minimally invasive vascular access solutions. A mid-bore venous closure device, so to speak, is a medical instrument engineered to close venous access sites made by mid-scale catheters or sheaths, supporting hemostasis and assisting in patient recovery after interventional processes. As a case in point, Haemonetics Corp broadened its VASCADE portfolio with the unveiling of the VASCADE MVP XL mid-bore venous closure device in June 2024. This device, fashioned for larger sheaths (10-12F, up to 15F exterior diameter) utilized in procedures such as cryoablation, includes 58% more collagen and a larger collapsible disc for speedy hemostasis. This medical marvel not only expands Haemonetics’ existing lineup of small- and mid-bore vascular closure solutions but also reinforces the firm’s industry-leading position in sophisticated closure technologies.
Secure Your Global Arteriotomy Closure Devices Market Report Now for Fast and Efficient Delivery!
https://www.thebusinessresearchcompany.com/report/arteriotomy-closure-devices-global-market-report
What Are the Key Components of the Arteriotomy Closure Devices Market, and How Do Its Segments Perform?
The arteriotomy closure devices market covered in this report is segmented –
1) By Product: Active Closure Devices, Passive Closure Devices
2) By Application: Femoral Arterial Access Procedures, Radial Arterial Access Procedures
3) By End User: Hospitals, Ambulatory Care Centers, Other End Users
Subsegments:
1) By Active Closure Devices: Suture-Based Devices, Staple-Based Devices, Vascular Closure Devices With Collagen Or Hemostatic Agents, Balloon-Based Devices
2) By Passive Closure Devices: Compression Devices, Hemostatic Patches Or Sponges, Vascular Plugs Or Plugs
Which Regions Are Most Influential in Expanding the Arteriotomy Closure Devices Market?
North America was the largest region in the arteriotomy closure devices market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the arteriotomy closure devices market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
What Does the Definition of the Arteriotomy Closure Devices Market Include?
An arteriotomy closure device (ACD) is a medical device designed to seal the puncture site in an artery following procedures such as catheterization or angiography. It facilitates hemostasis by closing the arterial opening, reducing bleeding risks, and promoting faster recovery compared to manual compression methods. ACDs are commonly used in minimally invasive vascular procedures to enhance patient safety and procedural efficiency.
Browse Through More Similar Reports By The Business Research Company:
Coronary Artery Disease Global Market Report 2025
https://thebusinessresearchcompany.com/report/coronary-artery-disease-global-market-report
Peripheral Artery Disease Global Market Report 2025
https://thebusinessresearchcompany.com/report/peripheral-artery-disease-global-market-report
Coronary Artery Bypass Graft Global Global Market Report 2025
https://thebusinessresearchcompany.com/report/coronary-artery-bypass-graft-global-market-report
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Contact us at:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at info@tbrc.info
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
Found this article helpful? Share it on: