Major Trends and Emerging Patterns in the Atrial Septal Defect Market: Advancements In Occluders For Arterial Septal Defect Treatment
Discover trends, market shifts, and competitive outlooks for the atrial septal defect industry through 2025-2034 with The Business Research Company’s reliable data and in-depth research
What Is the Current and Projected Market Size of the Atrial Septal Defect Market Through 2034?
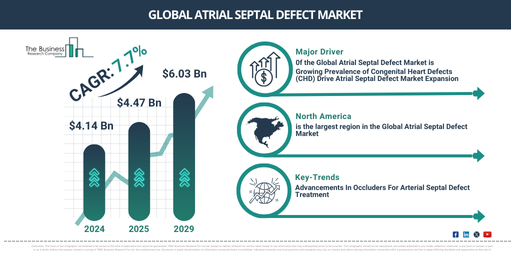
The market size for atrial septal defects has seen a robust growth in the past few years. The market is projected to go from $4.14 billion in 2024 to $4.47 billion in 2025, registering a compound annual growth rate (CAGR) of 8.0%. Multiple factors have contributed to this growth during the historic period, including an increase in the incidence of congenital heart disease, augmented funding for R&D related to ASD treatments, heightened awareness about congenital heart defects among parents and healthcare professionals, raised frequency of pediatric surgeries, authorization of novel ASD closure devices and therapies by regulatory authorities such as the FDA and the enlargement of healthcare facilities.
The market size of Atrial Septal Defect (ASD) is projected to experience a robust increase in the coming years. Its value is anticipated to reach $6.03 billion by 2029, with a compound annual growth rate (CAGR) of 7.7%. The expansion during the forecasted timeframe can be tied to factors such as a rise in the utilization of telemedicine for pre and post-surgery care, advancement in tailored treatment pathways built on genetic and phenotypic information, a consistent rise in worldwide healthcare expenditure, particularly within emerging markets, an aging demographic contributing to a rise in late-stage diagnoses of congenital heart anomalies. It also includes international alignment of medical device regulations that enhance global market penetration, and the execution of local and global health schemes aiming at early detection and management of congenital heart diseases. Key trends discerned during this period include the development of innovative, less invasive ASD closure apparatuses, the application of artificial intelligence (AI) and machine learning for superior diagnostics and personalized treatment strategies, the incorporation of biodegradable and bioresorbable resources in device production, AI-propelled devices for instant monitoring and decision-making support during ASD procedures, and the uptake of remote tracking technologies for patient recovery and early detection of complications.
Download a free sample to assess the report’s scope and structure:
https://www.thebusinessresearchcompany.com/sample.aspx?id=16345&type=smp
What Are the Primary Drivers Supporting the Market Growth of the Atrial Septal Defect Market?
The projected increase in congenital heart defects (CHD) incidents is likely to fuel the atrial septal defect market’s expansion. Such defects are structural anomalies in the heart, present from birth. There’s a noticeable rise in the number of individuals being diagnosed with these birth-present heart structure anomalies. Treatment of atrial septal defect proves pivotal in managing congenital heart defects by rectifying irregular blood circulation between the heart’s chambers, enhancing overall heart functionality, and mitigating related complications. As cited by The Australian Institute of Health and Welfare, a national agency of Australia, in June 2024 about 65,000 children and adults in Australia are living with congenital heart disease. There were around 5,900 hospital admissions in 2020–21 due to congenital heart disease being the main diagnosis, with 79 infants under one year old succumbing to the disease. This accounted for 7.8% of all infant fatality. Hence, the rising occurrences of congenital heart defects is expected to steer the atrial septal defect market onwards.
Which Primary Segments of the Atrial Septal Defect Market Are Driving Growth and Industry Transformations?
The atrial septal defect market covered in this report is segmented –
1) By Treatment Procedure: Surgical Closure, Transcatheter Closure, Hybrid Procedures, Medication Therapy
2) By Diagnosis: Chest X-Ray, Electrocardiogram, Cardiac Catheterization, Transesophageal Echocardiography, Pulse Oximetry
3) By Product Type: Medical Devices, Pharmaceutical Products
4) By Age Group: Pediatric, Adult
5) By End-User: Hospitals, Ambulatory Surgical Centers, Cardiac Clinics, Other End-Users
Subsegments:
1) By Surgical Closure: Open Heart Surgery (Traditional Surgical Closure), Minimally Invasive Surgical Closure, Patches (Pericardial, Synthetic) For Asd Closure
2) By Transcatheter Closure: Device-Based Closure (Amplatzer Septal Occluder), Transcatheter Plug Closure Techniques, Catheter-Based Asd Repair For Adult And Pediatric Patients
3) By Hybrid Procedures: Combination Of Surgical And Transcatheter Techniques, Minimally Invasive Hybrid Procedures, Hybrid Closure For Complex Or Large Asds
4) By Medication Therapy: Anticoagulant Therapy (To Prevent Stroke), Diuretics (For Managing Symptoms), Antihypertensive Drugs (For Managing Associated Conditions), Off-Label Use Of Other Medications
Request customized data on this market:
https://www.thebusinessresearchcompany.com/customise?id=16345&type=smp
Which Regions Are Key Players in the Growth of the Atrial Septal Defect Market?
North America was the largest region in the atrial septal defect market in 2023. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the atrial septal defect market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
Which Cutting-Edge Market Trends Are Expected to Drive the Atrial Septal Defect Market’s Growth?
The major players in the arterial septal defect market are striving to develop innovative devices and gain approval for them to expand their applications and accessibility. Acquiring device approval, generally from a regulatory authority or relevant body, is essential for the lawful manufacture, sale, or use of a device within a specific market or for a certain goal. For example, in March 2024, Occlutech GmbH, a prominent provider of minimally invasive cardiac devices based in Sweden, reported that the United States Food and Drug Administration (FDA) had granted device approval for its Occlutech ASD Occluder and Occlutech Pistol Pusher, used for treating atrial septal defects (ASD). This green signal from the FDA is a major breakthrough for the company in their ongoing efforts to improve global healthcare. The Occlutech ASD Occluder has been engineered to serve as a lifelong aid for patients with echocardiography-confirmed defects. It incorporates a self-expandable nitinol device featuring flexible discs that attach to both sides of the patient’s atrial septum, using the Occlutech Pistol Pusher. Post this approval, Occlutech will initiate its commercialization process in the US, exclusively in collaboration with B. Braun Interventional Systems.
View the full report here:
https://www.thebusinessresearchcompany.com/report/atrial-septal-defect-global-market-report
What Parameters Are Used to Define the Atrial Septal Defect Market?
An atrial septal defect (ASD) refers to a congenital heart defect characterized by an abnormal opening in the septum, the wall separating the heart’s two upper chambers (atria). This opening allows oxygen-rich blood from the left atrium to mix with oxygen-poor blood in the right atrium, which can lead to increased blood flow to the lungs and over time cause complications like pulmonary hypertension and heart failure.
Purchase the full report and get a swift delivery:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=16345
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Get in touch with us:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at info@tbrc.info
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
[KClientError] [REQ_ERR: OPERATION_TIMEDOUT] [KTrafficClient] Something is wrong.