Growth Trajectory Of The Bevacizumab Biosimilars Market 2024-2033
The Business Research Company’s global market reports are now updated with the latest market sizing information for the year 2024 and forecasted to 2033
The bevacizumab biosimilars market has shown significant growth in recent years and is poised for further expansion. This blog delves into the current market dynamics, future growth projections, and key trends driving this market.
Market Growth Overview
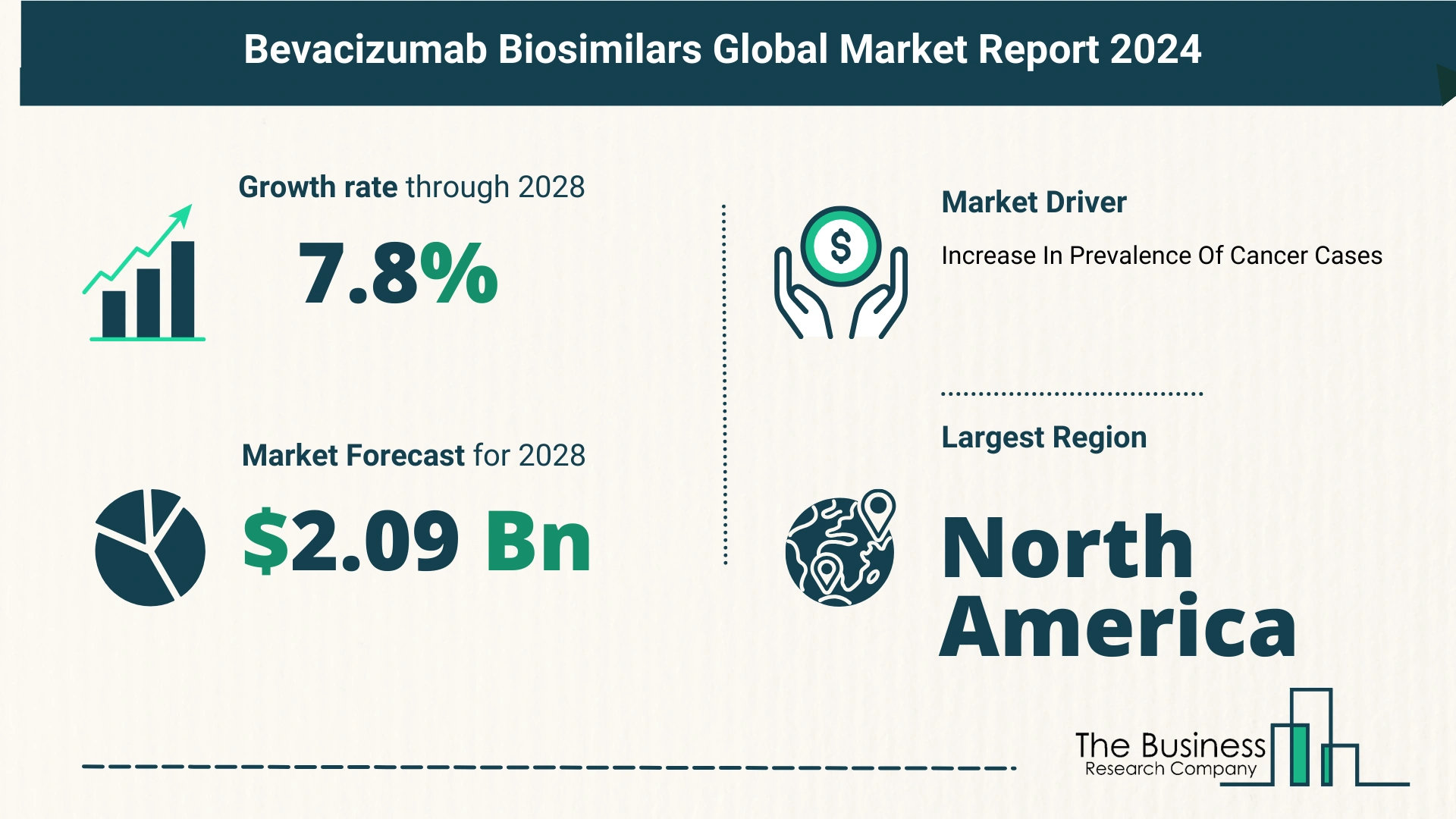
- 2023 Market Size: The market was valued at $1.43 billion in 2023.
- 2024 Projection: Expected to grow to $1.55 billion at a CAGR of 8.7%.
- 2028 Projection: Forecasted to reach $2.09 billion, maintaining a CAGR of 7.8%.
Key Drivers of Growth
Patent Expiry and Cost Containment
- Patent Expiry: The expiration of patents on original biologics has opened the market to biosimilars, providing cost-effective alternatives.
- Cost Containment: Healthcare systems globally are seeking to reduce costs, boosting the adoption of biosimilars.
Increasing Incidence of Cancer
- Rising Cancer Cases: The growing prevalence of cancer significantly drives the demand for bevacizumab biosimilars.
- Effectiveness: Bevacizumab biosimilars are crucial in treating cancers like non-small cell lung cancer and metastatic colorectal cancer.
Market Competitiveness and Accessibility
- Competition: Increased market competition leads to better pricing and accessibility.
- Patient Access: Enhanced affordability and availability of biosimilars improve patient access to essential cancer treatments.
Biosimilar Acceptance
- Regulatory Support: Growing acceptance and regulatory support for biosimilars enhance market growth.
View More On The Bevacizumab Biosimilars Market Report 2024 – https://www.thebusinessresearchcompany.com/report/bevacizumab-biosimilars-global-market-report
Future Market Trends
Rising Demand for Cancer Therapies
- Increased Need: The demand for effective cancer therapies is on the rise, driving the bevacizumab biosimilars market.
Expertise in Biosimilar Development
- Advanced R&D: Expertise in biosimilar development accelerates the introduction of new products.
Healthcare System Pressures
- Cost Pressures: Healthcare systems under financial strain increasingly adopt cost-effective biosimilars.
Regulatory Landscape and Interchangeability
- Regulatory Advancements: Improved regulatory frameworks support the approval and interchangeability of biosimilars, fostering market growth.
Major Trends Shaping the Future
Collaborations and Partnerships
- Strategic Alliances: Collaborations between companies enhance product development and market reach.
Regulatory Advancements and Approvals
- Streamlined Approvals: Regulatory advancements facilitate quicker and more efficient approvals for new biosimilars.
Innovation in Biosimilar Development
- Product Innovation: Companies are focusing on innovative biosimilars to strengthen their market positions.
Market Access Strategies and Lifecycle Management
- Access Strategies: Effective market access strategies ensure wider availability of biosimilars.
- Lifecycle Management: Managing the lifecycle of biosimilars helps maintain market relevance and competitiveness.
Growing Cancer Prevalence and Market Expansion
Increasing Cancer Cases
- Cancer Statistics: The rising number of cancer cases is a primary driver for the bevacizumab biosimilars market.
- Example: According to Macmillan Cancer Support, cancer patient numbers in the UK are expected to rise from 3 million in 2020 to 5.3 million by 2040.
Major Players in the Bevacizumab Biosimilars Market
- Leading Companies: Cipla Limited, Reliance Lifesciences Pvt. Ltd., Genentech Inc., Fujifilm Kyowa Kirin Biologics Co. Ltd., Pfizer Inc., AbbVie Inc., and others.
- Innovation: These companies are leading the way in biosimilar development and market expansion.
Advancing Product Innovation
Product Development
- New Biosimilars: Companies like Viatris and Biocon Biologics are launching innovative products like Abevmy, approved for multiple oncology indications.
Strategic Acquisitions
- Biocon and Viatris: Biocon Biologics’ acquisition of Viatris Inc.’s global biosimilars business in November 2022 strengthens its market presence.
Market Segmentation
By Product
- Products: Avastin, Mvasi, Zirabev, Aybintio, among others.
By Distribution Channel
- Channels: Hospital Pharmacy, Online Pharmacy, Retail Pharmacy, Other Distribution Channels.
By Application
- Cancer Types: Colorectal Cancer, Non-small Cell Lung Cancer, Glioblastoma, Renal Cell Carcinoma, Cervical Cancer, Ovarian Cancer.
Regional Insights
North America
- Largest Market: North America was the largest region in the bevacizumab biosimilars market in 2023.
Conclusion
The bevacizumab biosimilars market is on a robust growth trajectory, driven by rising cancer prevalence, cost containment needs, and increased biosimilar acceptance. With ongoing innovation, strategic collaborations, and supportive regulatory frameworks, the market is well-positioned for continued expansion in the coming years.
Request A Sample Of The Global Bevacizumab Biosimilars Market Report 2024:
https://www.thebusinessresearchcompany.com/sample_request?id=10848&type=smp