What Is The Forecast Growth Rate For The Biologic And Biosimilar RA Drug Market?
The Business Research Company’s market reports offer an in-depth analysis on the market’s growth potential, major drivers, key trends and more.
Dynamics of Growth
- The market size of biologic & biosimilar RA drugs has witnessed robust expansion.
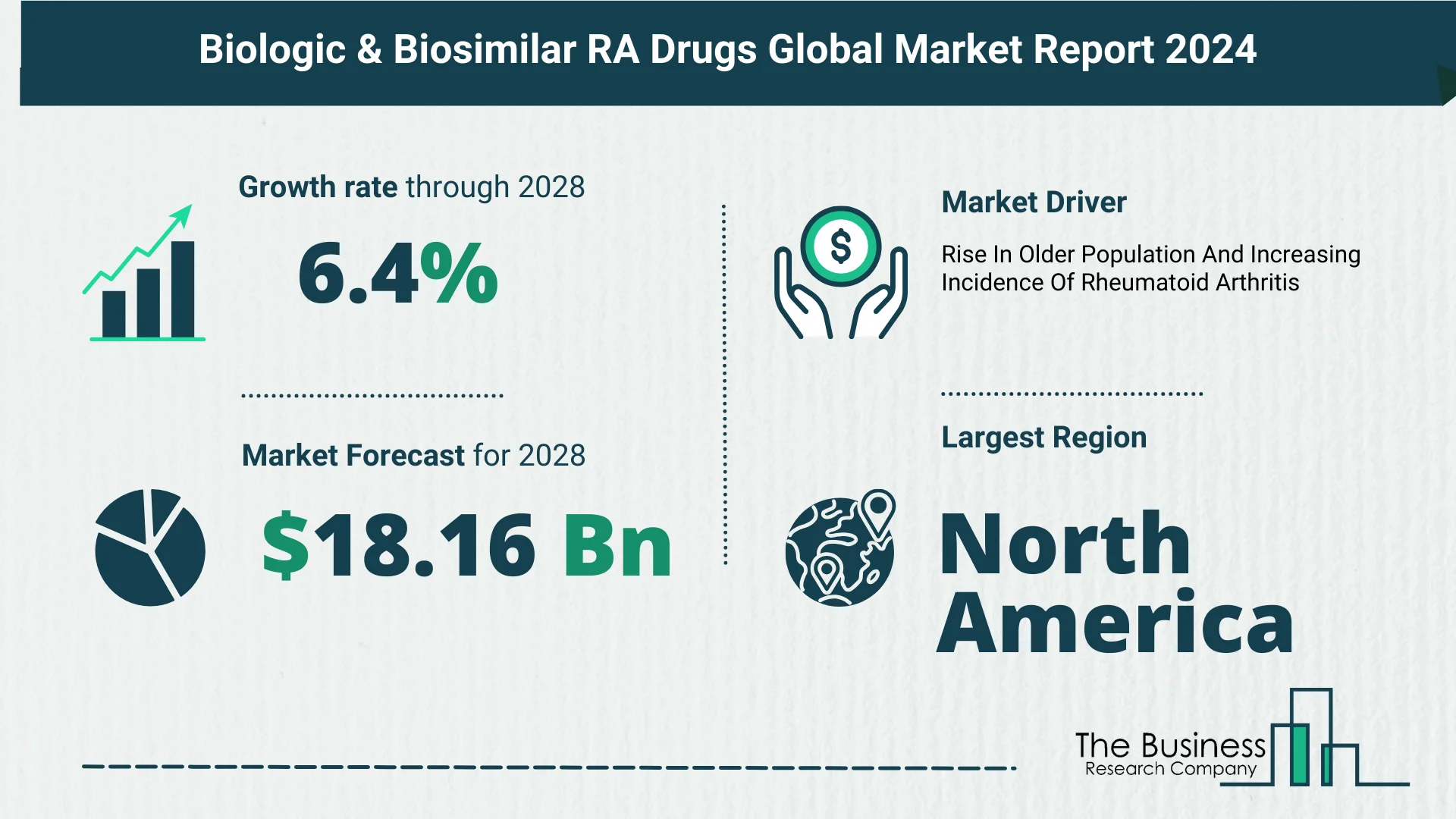
- From $13.44 billion in 2023, it is projected to reach $14.15 billion in 2024, boasting a 5.3% compound annual growth rate (CAGR).
- Historical growth is attributed to factors like market adoption rates, clinical trial outcomes, and pricing strategies.
Embracing Future Potential
- Forecasts indicate even stronger growth, with the market set to soar to $18.16 billion by 2028, boasting a 6.4% CAGR.
- Anticipated growth factors include increasing biologic drug development, evolving healthcare policies, and expanding patient preferences.
Emerging Trends Shaping the Landscape
- Rise in Biosimilar Market Competition: Intensifying competition promises more affordable treatment options.
- Personalized Medicine and Precision Therapy: Tailored approaches enhance treatment efficacy and patient outcomes.
- Advancements in Drug Delivery Systems: Innovations optimize drug administration and improve patient compliance.
- Focus on Safety and Efficacy: Enhanced drug safety profiles prioritize patient well-being.
- Patient-Centric Healthcare: Patient preferences and needs increasingly drive treatment decisions.
Geriatric Population Surge and RA Incidence
- The growing geriatric population and rising incidence of rheumatoid arthritis fuel market growth.
- Rheumatoid arthritis, an autoimmune disease, disproportionately affects older individuals.
- Biologics and biosimilar RA drugs offer superior treatment options compared to conventional drugs.
- Statistics project a significant increase in arthritis cases, underlining the market’s potential for expansion.
Noteworthy Innovations
- Major companies introduce innovative biologics for polymyalgia rheumatica treatment.
- Regeneron Pharmaceuticals, Inc. and Sanofi’s FDA-approved Kevzara offers new hope for PMR patients.
- Positive outcomes from the SAPHYR Phase 3 trial showcase Kevzara’s efficacy in sustaining remission.
Strategic Acquisitions and Expansions
- Biocon’s acquisition of Viatris’ global biosimilars business for $3 billion expands its biologics portfolio.
- The acquisition strengthens Biocon’s commercial capabilities across emerging and advanced markets.
- Viatris’ expertise in manufacturing biosimilars augments Biocon’s position in the market.
Market Segmentation Insights
- By Source: Microbial, Mammalian, Other Sources.
- By Disease: Oncology, Immunological Disorders, Cardiovascular Disorders, Hematological Disorders, Other Diseases.
- By Manufacturing: Outsourced, In-House.
- By Mode of Purchase: Prescription Drugs, Over-The-Counter (OTC) Drugs.
- By Distribution Channel: Hospital Pharmacies, Retail Pharmacies, Online Pharmacies.
Regional Dynamics
- North America leads the biologic & biosimilar RA drugs market in 2023.
- Asia-Pacific emerges as the fastest-growing region during the forecast period.
The biologic & biosimilar RA drugs market presents a dynamic landscape driven by innovation, strategic partnerships, and evolving patient needs. As the market continues to evolve, stakeholders navigate through opportunities and challenges, ensuring access to effective treatments for individuals battling rheumatoid arthritis and related conditions.
Read More On The Biologic And Biosimilar RA Drug Market Report 2024 – https://www.thebusinessresearchcompany.com/report/biologic-and-biosimilar-ra-drugs-global-market-report
Request for A Sample Of The Global Biologic And Biosimilar RA Drug Market Report:
https://www.thebusinessresearchcompany.com/sample_request?id=9255&type=smp