Global Cell Free DNA (cfDNA) Testing Market Key Insights 2024-2033
The Business Research Company’s global market reports are now updated with the latest market sizing information for the year 2024 and forecasted to 2033
Introduction: A Rapidly Growing Sector
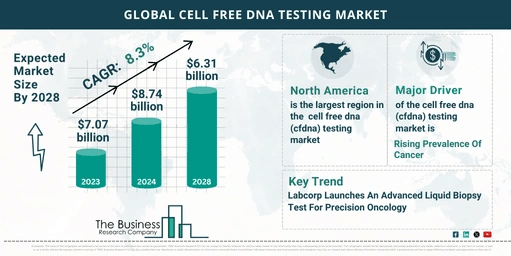
The cell-free DNA (cfDNA) testing market is experiencing remarkable growth, driven by advancements in technology and increasing medical applications. The market size surged from $7.07 billion in 2023 to $8.74 billion in 2024, reflecting a robust compound annual growth rate (CAGR) of 23.6%. This growth trajectory highlights the expanding role of cfDNA testing in modern healthcare.
Key Drivers of Growth
- Increased Adoption in Prenatal Care: cfDNA testing has become a critical tool in prenatal care, providing early detection of genetic disorders with minimal risk to the mother and fetus.
- Advancements in cfDNA Analysis: Enhanced analytical techniques are broadening the scope of cfDNA testing, making it applicable across various medical fields.
- Regulatory Approvals: Growing approvals from regulatory bodies have facilitated the wider use of cfDNA tests, ensuring their reliability and efficacy.
Future Market Projections
The cfDNA testing market is projected to grow to $20.63 billion by 2028, with a CAGR of 23.9%. This continued expansion is fueled by several factors:
- Personalized Medicine: The shift towards personalized medicine emphasizes the need for precise diagnostic tools like cfDNA testing.
- Healthcare Infrastructure: Improvements in healthcare infrastructure are supporting the adoption of advanced diagnostic technologies.
- Aging Population and Cancer Incidence: The growing aging population and rising cancer rates are increasing the demand for early and accurate diagnostic methods.
View More On The Cell Free DNA (cfDNA) Testing Market Report 2024 – https://www.thebusinessresearchcompany.com/report/cell-free-dna-cfdna-testing-global-market-report
Trends Shaping the Market
- Liquid Biopsy Technologies: Adoption of liquid biopsy techniques is enhancing the sensitivity and specificity of cancer detection.
- Next-Generation Sequencing (NGS): Advancements in NGS technology are enabling more detailed genetic analysis.
- AI and Machine Learning: Integration of AI and machine learning is improving data interpretation and diagnostic accuracy.
- Point-of-Care Testing: Development of point-of-care cfDNA tests offers convenience and rapid results.
- Cloud-Based Platforms: Implementation of cloud-based platforms for data storage and analysis is facilitating better data management and accessibility.
The Impact of Rising Cancer Prevalence
The increasing prevalence of cancer is a significant driver of cfDNA testing market growth. cfDNA tests can detect genetic mutations associated with cancer at an early stage, often before symptoms appear. This early detection allows for timely intervention and improved patient outcomes.
- Cancer Statistics: According to the World Health Organization (WHO), the number of new cancer cases is expected to rise from 20 million in 2022 to over 35 million by 2050.
- Early Intervention: cfDNA testing aids in identifying cancer in its initial stages, enhancing treatment efficacy and survival rates.
Innovations by Major Companies
Labcorp Launches Advanced Liquid Biopsy Test
- Product Launch: In May 2023, Laboratory Corporation of America Holdings (Labcorp) introduced Labcorp Plasma Focus, a liquid biopsy test designed for patients with advanced or metastatic solid tumors.
- Benefits: This test, which requires only a standard blood draw, enhances precision oncology by identifying actionable biomarkers across various cancers.
LabCorp Enhances Capabilities Through Acquisition
- Strategic Acquisition: In February 2022, Labcorp acquired Personal Genome Diagnostics Inc., a leader in precision oncology diagnostics.
- Objective: The acquisition aims to advance Labcorp’s liquid biopsy capabilities and expand its genomic profiling tools for cancer.
Market Segmentation
The cfDNA testing market is segmented into:
- By Product:
- Donor-Derived Cell-Free DNA
- Circulating Cell-Free Tumor DNA
- Cell-Free Fetal DNA
- By Platforms:
- Next Generation Sequencing (NGS)
- rPCR and Multiplexed PCR
- qPCR and dPCR
- Other Platforms
- By Application:
- Oncology
- Non-Invasive Prenatal Testing (NIPT)
- Gynecology
- Transplantation
- Other Applications
Regional Insights
- North America: Held the largest share of the cfDNA testing market in 2023.
- Asia-Pacific: Expected to be the fastest-growing region due to increasing healthcare investments and rising awareness.
Conclusion
The cell-free DNA testing market is on a dynamic growth path, driven by technological innovations, increasing cancer prevalence, and a shift towards personalized medicine. With ongoing advancements and a broadening application scope, cfDNA testing is set to play an increasingly vital role in modern healthcare.
Request A Sample Of The Global Cell Free DNA (cfDNA) Testing Market Report 2024:
https://www.thebusinessresearchcompany.com/sample_request?id=15757&type=smp