Key Takeaways From The Global Clinical Trial Equipment And Ancillary Solutions Market Forecast 2024
The Business Research Company’s market reports offer an in-depth analysis on the market’s growth potential, major drivers, key trends and more.
Current Market Dynamics
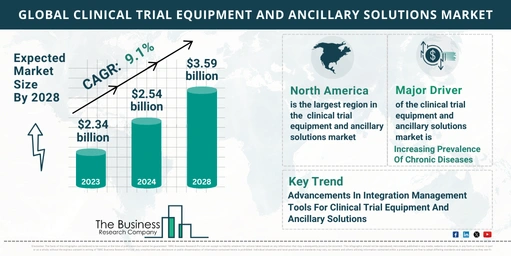
- Market Size: The market increased from $2.34 billion in 2023 to $2.54 billion in 2024.
- Growth Rate: This represents a compound annual growth rate (CAGR) of 8.7%.
- Historical Drivers:
- Rising prevalence of chronic diseases.
- Increasing research expenditure.
- Expansion in the pharmaceutical and medical sectors.
- Growing emphasis on patient-centric clinical trials.
Future Market Projections
- Projected Growth: The market is expected to reach $3.59 billion by 2028.
- Forecast CAGR: Reflects a CAGR of 9.1%.
- Future Drivers:
- Continued rise in chronic diseases.
- Increasing spending on research.
- Expansion of the pharmaceutical and medical sectors.
- Growth in patient-centric clinical trials.
Read More On The Clinical Trial Equipment And Ancillary Solutions Market Report 2024 –
https://www.thebusinessresearchcompany.com/report/clinical-trial-equipment-and-ancillary-solutions-global-market-report
Impact of Chronic Diseases
- Market Growth: The increasing prevalence of chronic diseases is a significant driver for the clinical trial equipment and ancillary solutions market.
- Chronic Diseases:
- Conditions such as cancer, diabetes, and cardiovascular diseases are becoming more prevalent due to lifestyle changes, environmental factors, and genetic predispositions.
- Clinical trial equipment and ancillary solutions are critical in managing these diseases by enabling early detection, personalized treatment approaches, and improved monitoring and outcomes.
- Example: In January 2024, the Australian Institute of Health and Welfare reported that chronic kidney disease contributed to approximately 20,000 fatalities in Australia in 2021, representing 12% of the total mortality rate.
Technological Advancements
- Integration Platforms: Companies are focusing on developing integration platforms to enhance workflow efficiency across clinical trial systems and technologies.
- Example: In March 2022, Vibrent Health developed the Ancillary Studies Tool Suite as part of its Digital Health Research Platform, designed to seamlessly integrate digital apps, sensors, and data collection platforms into longitudinal cohort research, optimizing workflow and enhancing collaboration.
Strategic Acquisitions
- Thermo Fisher Scientific’s Acquisition of PPD Inc.: In December 2021, Thermo Fisher Scientific acquired PPD Inc. for $17.4 billion to expand its clinical development portfolio.
- Purpose: The acquisition enables Thermo Fisher to offer a comprehensive suite of services across the clinical development spectrum, from safety assessments to clinical trial logistics and drug development.
- PPD Inc.: A US-based company specializing in clinical trial equipment and ancillary solutions, enhancing Thermo Fisher’s capabilities in this space.
Market Segmentation
- By Type:
- Diagnostic Equipment
- Laboratory Instruments
- Ancillary Solutions
- By Service:
- Rental and Leasing Services
- Supply and Logistics Services
- Regulatory Compliance Services
- Software Services
- By End-User:
- Pharmaceutical and Biotechnology Companies
- Contract Research Organizations (CROs)
- Medical Device Companies
- Others
Regional Insights
- North America: The largest region in the clinical trial equipment and ancillary solutions market in 2023.
- Asia-Pacific: Expected to be the fastest-growing region during the forecast period.
The clinical trial equipment and ancillary solutions market is poised for strong growth, driven by the rising prevalence of chronic diseases, increasing research spending, and technological advancements. The market’s expansion is further supported by strategic acquisitions and the development of innovative integration platforms, which are expected to enhance workflow efficiency and collaboration in clinical trials.
Request for A Sample Of The Global Clinical Trial Equipment And Ancillary Solutions Market Report:
https://www.thebusinessresearchcompany.com/sample_request?id=15764&type=smp