Clinical Trial Equipment And Ancillary Solutions Outlook 2034: Key Drivers, Trends, and Market Frontiers
Discover trends, market shifts, and competitive outlooks for the clinical trial equipment and ancillary solutions industry through 2025-2034 with The Business Research Company’s reliable data and in-depth research
How Has The Clinical Trial Equipment And Ancillary Solutions Market Size Shifted, And What Is the Outlook Through 2034?
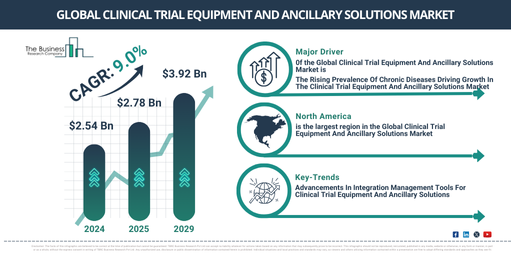
The market for clinical trial equipment and ancillary solutions has experienced robust growth in the past few years. The market value is projected to increase from $2.54 billion in 2024 to about $2.78 billion in 2025, featuring a compound annual growth rate (CAGR) of 9.3%. Factors contributing to this rise during the historic period include an increase in chronic diseases, higher expenditure on research, development in the pharmaceutical industry, the expansion of medical sectors, and an increase in patient-centric clinical trials.
The size of the market for clinical trial equipment and ancillary solutions is projected to exhibit robust growth in the coming years. Predictions estimate that it will reach $3.92 billion by 2029, with a compound annual growth rate (CAGR) of 9.0%. This growth during the forecast period is likely due to the escalating prevalence of chronic diseases, increased research expenditure, the expansion of the pharmaceutical and medical sectors, and the rise of patient-centric clinical trials. The forecast period also anticipates significant trends such as technological progress, advancements in ancillary solutions, the evolution of developmental services, the creation of innovative equipment, and the development of inventive therapies.
Download a free sample to assess the report’s scope and structure:
https://www.thebusinessresearchcompany.com/sample.aspx?id=15764&type=smp
How Are Key Drivers in the Industry Acting as Catalysts for the Growth of the Clinical Trial Equipment And Ancillary Solutions Market?
The escalating incidence of chronic ailments is anticipated to fuel the expansion of the market for clinical trial equipment and ancillary solutions. Such chronic maladies are typically enduring conditions that necessitate continuous medical care and may impede daily activities or life quality. The escalation in chronic diseases can be attributed to factors like air pollution, toxic substances, lifestyle modifications, and genetic makeup. The role of clinical trial equipment and ancillary solutions in improving results for people dealing with chronic disorders is significant, as these tools facilitate early diagnosis, individualized treatment plans, objective evaluation of treatment success, safety surveillance, patient involvement, enhanced trial configuration, adherence to regulations, and collaborative research. In an instance, the National Center for Biotechnology Information (NCBI), a national library of medicine based in the US, projected in January 2023 that by 2050, people aged 50 years and above diagnosed with at least one chronic disease will skyrocket by 99.5%, escalating from 71.522 million in 2020 to 142.66 million. Therefore, this upsurge in chronic diseases is seen to power the growth of the clinical trial equipment and ancillary solutions market.
Which Segments in the Clinical Trial Equipment And Ancillary Solutions Offer the Most Growth?
The clinical trial equipment and ancillary solutions market covered in this report is segmented –
1) By Type: Diagnostic Equipment, Laboratory Instruments, Ancillary Solutions
2) By Service: Rental And Leasing Services, Supply And Logistics Services, Regulatory Compliance Services, Software Services
3) By End-User: Pharmaceutical And Biotechnology Companies, Contract Research Organizations (CROs), Medical Device Companies, Others End-Users
Subsegments:
1) By Diagnostic Equipment: Imaging Devices, Electrocardiogram (ECG) Machines, Blood Pressure Monitors, Pulse Oximeters
2) By Laboratory Instruments: Centrifuges, Spectrometers, Microscopes, Pipettes And Dispensers
3) By Ancillary Solutions: Clinical Trial Management Software (CTMS), Patient Recruitment Solutions, Data Management Solutions, Temperature Control Systems
Request customized data on this market:
https://www.thebusinessresearchcompany.com/customise?id=15764&type=smp
What Are the Fastest-Growing Geographies in the Clinical Trial Equipment And Ancillary Solutions Market?
North America was the largest region in the clinical trial equipment and ancillary solutions market in 2023. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the clinical trial equipment and ancillary solutions market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa.
What Key Market Trends and Innovations Are Shaping the Future of the Clinical Trial Equipment And Ancillary Solutions Industry?
Key players in the clinical trial equipment and ancillary solutions market are working on creating integration platforms to improve efficiency and overall workflow across diverse clinical trial systems and technologies. This centralized platform acts as a linkage point, combining various tools, technologies, and services required in clinical research. It promotes data and information exchange among different elements of the clinical trial ecosystem, thereby enhancing workflows, augmenting efficiency, and fostering collaboration among entities participating in clinical trials. As an example, in March 2022, a healthcare firm based in the US, Vibrent Health, revealed that they are developing an Ancillary Studies Tool Suite, part of its Digital Health Research Platform. This suite aims to offer crucial integration management tools to effortlessly encompass existing digital apps, sensors, and data collection platforms into longitudinal cohort studies.
View the full report here:
What Are the Key Elements That Define the Clinical Trial Equipment And Ancillary Solutions Market?
Clinical trial equipment and ancillary solutions refer to a broad range of products, services, and support systems utilized in clinical research to facilitate the planning, execution, and management of clinical trials. These solutions are necessary for conducting safe, efficient, and compliant clinical studies across different therapeutic areas and phases of drug development. The clinical trial equipment and ancillary solutions support the successful planning, execution, and completion of clinical trials, ultimately contributing to the advancement of medical science and improved patient care.
Purchase the full report and get a swift delivery:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=15764
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Get in touch with us:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at info@tbrc.info
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
[KClientError] [REQ_ERR: OPERATION_TIMEDOUT] [KTrafficClient] Something is wrong.