Overview Of The Clinical Trial Management System (CTMS) Market 2024-2033: Growth And Major Players Analysis
The Business Research Company’s global market reports are now updated with the latest market sizing information for the year 2024 and forecasted to 2033
- Market Growth Overview:
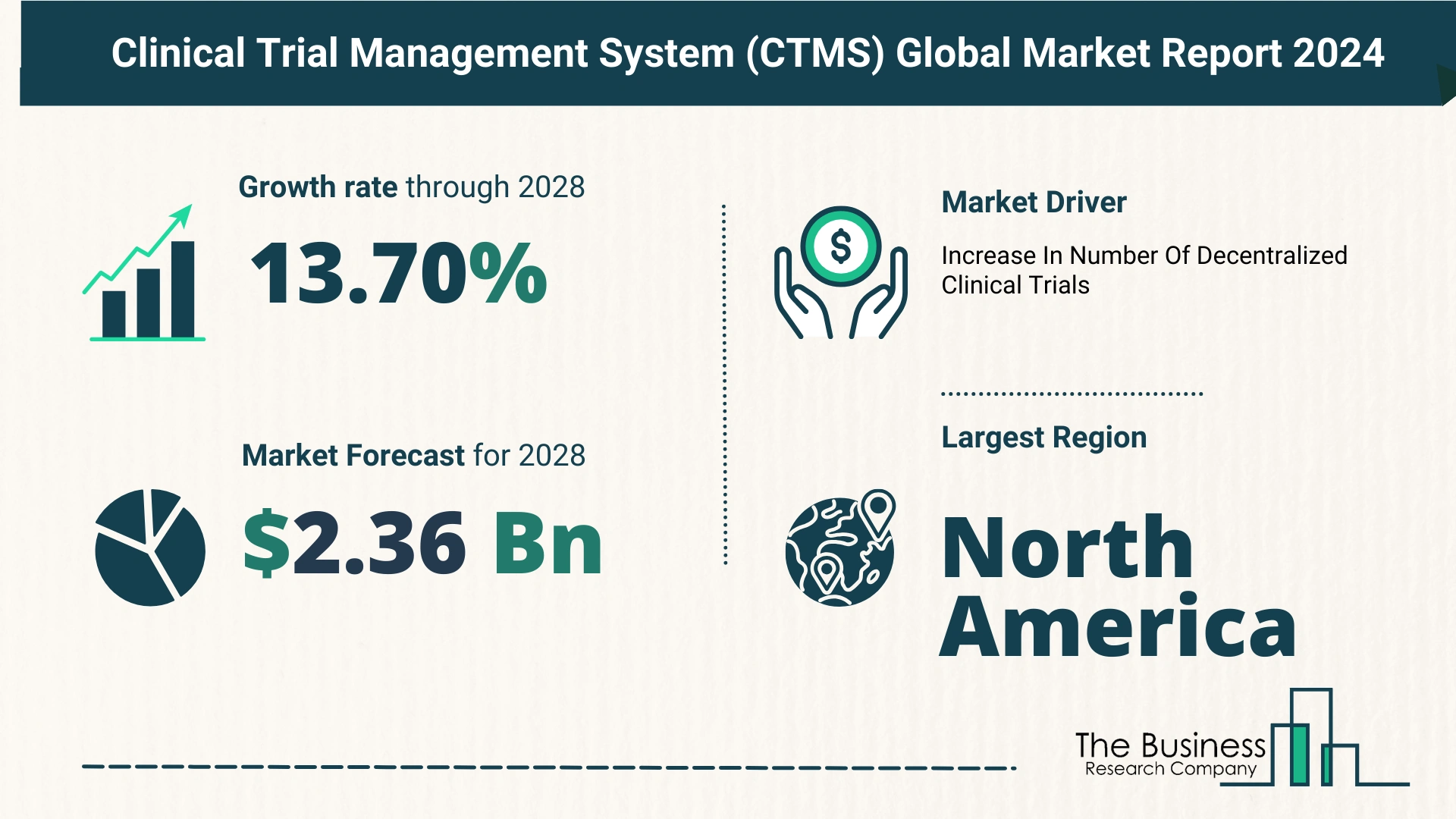
- From $1.23 billion in 2023 to $1.41 billion in 2024.
- Compound Annual Growth Rate (CAGR) of 15.3%.
- Factors Driving Growth:
- Increasing clinical trial complexity.
- Rise in global clinical trials.
- Stringent regulatory compliance.
- Growing focus on patient-centric trials.
- Increasing outsourcing of clinical trials.
Anticipated Growth and Trends in CTMS
- Projected Growth:
- $2.36 billion by 2028.
- CAGR of 13.7%.
- Drivers of Forecasted Growth:
- Emphasis on real-time data access.
- Integration with Electronic Health Records (EHRs).
- Rise in virtual and decentralized trials.
- Focus on risk-based monitoring.
- Advancements in analytics and AI.
- Major Trends:
- Focus on regulatory compliance and data security.
- Rise of adaptive clinical trial designs.
- Collaborative platforms for stakeholder engagement.
- Remote site monitoring and management.
- Evolving data standards and interoperability.
Adoption of SaaS and AI Integration in CTMS Solutions for Enhanced Clinical Trial Efficiency
- Decentralized Clinical Trials Driving Market Growth:
- Utilization of telemedicine and local healthcare services.
- Monitoring recruitment and participant activities.
- Increasing number of decentralized clinical trials.
- Innovations by Major Companies:
- Development of SaaS-based CTMS.
- Integration with artificial intelligence.
- Example: Saama Technologies’ unified platform.
View More On The Clinical Trial Management System (CTMS) Market Report 2024 – https://www.thebusinessresearchcompany.com/report/clinical-trial-management-system-ctms-global-market-report
Advarra Strengthens Clinical Research Offerings Through Acquisition of Bio-Optronics
- Strategic Acquisition:
- Advarra Inc. acquires Bio-Optronics.
- Enhancing capabilities for research institutions.
- Streamlining and optimizing clinical research processes.
Market Segmentation
- By System Type:
- Enterprise Clinical Trial Management System.
- On-Site Clinical Trial Management System.
- By Component:
- Software.
- Service.
- Hardware.
- By Delivery:
- Web-based (On-demand).
- Licensed Enterprise (On-premises).
- Cloud-based (SaaS).
- By End User:
- Large Pharma-biotech Companies.
- CROs.
- Medical Device Manufacturers.
- Small And Mid-sized Pharma-biotech Companies.
- Other End Users.
Regional Insights
- North America:
- Largest region in the CTMS market in 2023.
- Asia-Pacific:
- Expected to be the fastest-growing region in the forecast period.
In conclusion, the clinical trial management system (CTMS) market is experiencing significant growth, driven by factors like increasing trial complexity and advancements in technology. The adoption of SaaS and AI integration is poised to enhance efficiency in clinical trials, while strategic acquisitions like Advarra’s acquisition of Bio-Optronics further strengthen the industry’s capabilities. With evolving trends and regional dynamics, the CTMS market continues to expand, offering promising opportunities for stakeholders across the globe.
Request A Sample Of The Global Clinical Trial Management System (CTMS) Market Report 2024:
https://www.thebusinessresearchcompany.com/sample_request?id=13147&type=smp