Key Developments and Trends Steering the Clinical Trial Management System (CTMS) Market Forward: Growing Embrace Of SAAS And AI Integration In CTMS Solutions To Streamline Clinical Trials
Discover trends, market shifts, and competitive outlooks for the clinical trial management system (ctms) industry through 2025-2034 with The Business Research Company’s reliable data and in-depth research
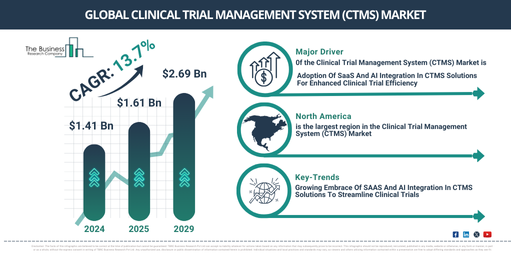
What is the Anticipated CAGR of the Clinical Trial Management System (CTMS) Market, and What Factors Will Drive It?
The market size of the clinical trial management system (CTMS) has witnessed substantial growth in the past years. The rise from $1.41 billion in 2024 to $1.61 billion in 2025, showcasing a compound annual growth rate (CAGR) of 14.2%, is quite remarkable. The historical growth trends are mainly due to factors such as increased complexity in clinical trials, a surge in global clinical trials, strict regulatory compliance, more emphasis on patient-centric trials, and the escalated outsourcing of clinical trials.
The market size for the clinical trial management system (CTMS) is projected to witness a substantial increment in the forthcoming years. The market is anticipated to expand to $2.69 billion by 2029, augmenting at a compound annual growth rate (CAGR) of 13.7%. The significant expansion in the forecast period can be connected to the constant requirement for real-time data exposure, harmonization with electronic health records (EHRS), an increase in the execution of virtual and decentralized trials, more commitment toward risk-based supervision, and progress in analytics and artificial intelligence. Key trends during this forecast period incorporate a concentrated attention on regulatory compliance and data protection, the rising popularity of adaptive clinical trial models, cooperative platforms for stakeholder involvement, remote monitoring and handling of sites, and evolving standards and interoperability of data.
Download a free sample to assess the report’s scope and structure:
https://www.thebusinessresearchcompany.com/sample.aspx?id=13147&type=smp
How Are Key Drivers in the Industry Acting as Catalysts for the Growth of the Clinical Trial Management System (CTMS) Market?
The projected expansion of the clinical trial management system (CTMS) market is largely attributed to the growing prevalence of decentralized clinical trials. These trials differ from conventional approaches as they incorporate telemedicine and local healthcare services, thus eliminating or lessening the requirement for patients to attend hospital-based trial sites. A CTMS closely tracks enrollment and participant activities and renders comprehensive supervision and documentation throughout these decentralized processes. As reported by the UK’s Clinical Trials Arena in February 2023, there is an anticipated 17% surge in the adoption of decentralization components in clinical trials by year-end 2023, surpassing the record numbers recorded in 2021. Consequently, the amplification in decentralized clinical trials is set to stimulate the evolution of the CTMS market. The Clinical Trial Management System (CTMS) Market will be influenced significantly by the escalating demand for personalized medicine. Personalized medicine is an approach that utilizes a patient’s genetic makeup to inform decisions pertaining to disease prevention, diagnosis, and cure. The application of CTMS can customize the clinical trial procedures, modifying recruitment, protocol formation, data compilation, patient involvement and data scrutiny to cater to the individual requirements of each patient. As per the Personalized Medicine Coalition’s Scope and Significance of Progress report in 2022, the US FDA sanctioned 12 personalized medicines in 2022, which constituted roughly 34% of all freshly approved molecular entities for treatment. As a result, the augmented demand for personalized medicine fuels the expansion of the CTMS market.
Which Segments in the Clinical Trial Management System (CTMS) Offer the Most Growth?
The clinical trial management system (CTMS) market covered in this report is segmented –
1) By System Type: Enterprise Clinical Trial Management System, On-Site Clinical Trial Management System
2) By Component: Software, Service, Hardware
3) By Delivery: Web-Based (On-Demand), Licensed Enterprise (On-Premises), Cloud-Based (SaaS)
4) By End User: Large Pharma-Biotech Companies, CROs, Medical Device Manufacturers, Small And Mid-Sized Pharma-Biotech Companies, Other End Users
Subsegments:
1) By Enterprise Clinical Trial Management System: Cloud-Based Solutions, On-Premises Solutions
2) By On-Site Clinical Trial Management System: Manual Systems, Semi-Automated Systems
Request customized data on this market:
https://www.thebusinessresearchcompany.com/customise?id=13147&type=smp
What Are the Fastest-Growing Geographies in the Clinical Trial Management System (CTMS) Market?
North America was the largest region in the clinical trial management system (CTMS) market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the clinical trial management system (CTMS) market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa
What Are the Current Market Growth and Trends in the Clinical Trial Management System (CTMS) Industry?
Leading firms in the clinical trial management system (CTMS) market are introducing innovative tech platforms such as Software as a Service (SaaS) integrated with artificial intelligence to accelerate pharmaceutical development. SaaS-based CTMS is a cloud-based software that aids in effectively managing and monitoring different aspects of clinical trials, making it accessible from anywhere that has an internet connection, which eliminates the requirement for local software installation and upkeep. Take for example, in June 2023, Saama Technologies, Inc., a US company specializing in clinical development software and services, introduced a combined platform of SaaS-based products that enhance its current AI and machine learning solutions. This helps in quicker clinical development and commercialization. This platform streamlines crucial clinical trial management operations, decreases query generation times by up to 90%, and brings significant time efficiency in study data transformation and analysis. It provides a complete overview of trial procedures and patient progress from a singular location.
View the full report here:
What Are the Key Elements That Define the Clinical Trial Management System (CTMS) Market?
A clinical trial management system (CTMS) is a software platform designed to facilitate and streamline the management of clinical trials. It is a comprehensive solution to centralize, organize, and automate various aspects of the clinical trial process, enabling efficient study planning, execution, monitoring, and reporting.
Purchase the full report and get a swift delivery:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=13147
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Get in touch with us:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at info@tbrc.info
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model