Clinical Trial Supplies Global Market Outlook 2024-2033: Size And Growth Rate Analysis
The Business Research Company’s global market reports provide comprehensive analysis on the various markets in 27 industries across 60 geographies.
Understanding the Market Dynamics
The clinical trial supplies market has witnessed significant growth in recent years, driven by various factors:
- Rising Prevalence of Chronic Diseases: The increasing incidence of chronic illnesses necessitates extensive clinical trials for developing new treatments.
- Stringent Regulatory Requirements: Compliance with regulatory standards necessitates robust clinical trial supply management.
- Growing Demand for Clinical Trials: There’s a heightened demand for clinical trials to address evolving medical needs and innovations.
- Increasing Complexity of Clinical Trials: Trials are becoming more intricate, requiring diverse and specialized supplies.
Current Market Scenario
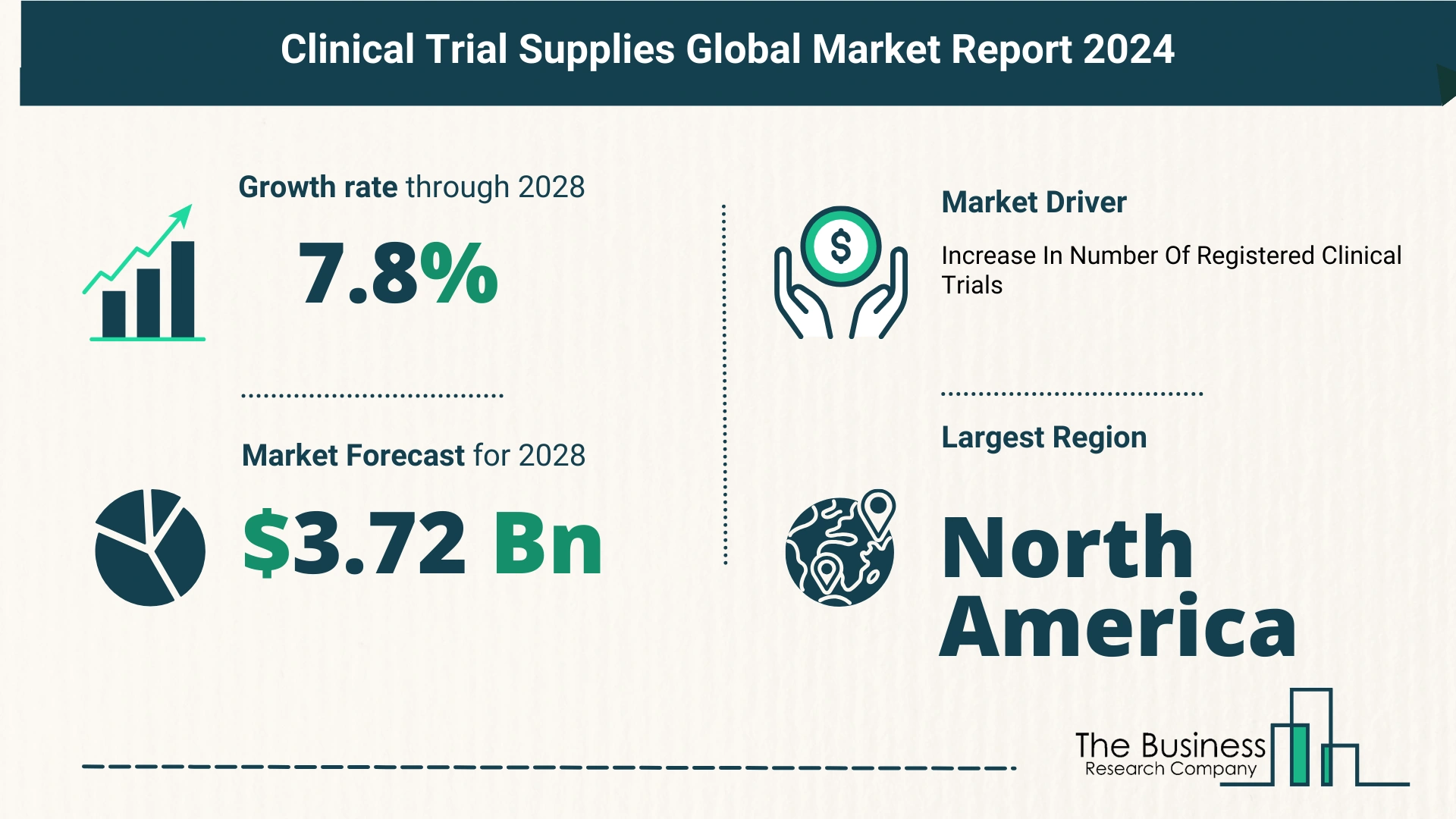
The market size surged from $2.52 billion in 2023 to $2.75 billion in 2024, indicating a Compound Annual Growth Rate (CAGR) of 8.9%.
Factors Driving Future Growth
Globalization of Clinical Trials
- Expansion of Cell and Gene Therapy Trials: The emergence of advanced therapies fuels the demand for clinical trial supplies.
- Focus on Patient-Centric Trials: Enhancing patient experience and participation reshapes trial methodologies.
- Technological Advancements: Integration of AI, 3D printing, and blockchain streamlines supply chain management.
View More On The Clinical Trial Supplies Market Report 2024 – https://www.thebusinessresearchcompany.com/report/clinical-trial-supplies-global-market-report
Industry Innovations and Trends
Incorporation of Real-World Evidence Platforms
- Leveraging Real-World Data: Companies harness real-world data to derive actionable insights, shaping decision-making.
- Example of Komodo Health: The launch of MapEnhance demonstrates the integration of real-world evidence into clinical trial strategies.
Strategic Acquisitions Driving Market Expansion
Thermo Fisher Scientific’s Acquisition of PPD Inc.
- Strategic Expansion: Thermo Fisher’s acquisition of PPD Inc. broadens its clinical development capabilities and service offerings.
Market Segmentation
- By Services: Logistics, storage, supply chain management, packaging, labeling, manufacturing, and comparator sourcing.
- By Clinical Phases: Segmented into Phase I, II, III, and IV trials.
- By Therapeutic Use: Including oncology, CNS, cardiovascular, infectious disease, metabolic disorders, and other therapeutic areas.
- By End User: Pharmaceutical and biotech companies, contract research organizations, and medical device firms.
Regional Insights
- Dominance of North America: North America led the market in 2023, driven by robust research infrastructure and investments.
- Emerging Growth in Asia-Pacific: Asia-Pacific is poised to witness rapid growth, fueled by increasing clinical trial activities and evolving healthcare landscape.
Conclusion
The clinical trial supplies market is undergoing significant expansion, fueled by the burgeoning demand for innovative treatments and therapies. As the industry embraces technological advancements and incorporates real-world evidence, it paves the way for more efficient and patient-centric clinical trials. With strategic acquisitions and a focus on sustainability, the market is poised for sustained growth in the coming years, offering ample opportunities for stakeholders across the globe.
Request A Sample Of The Global Clinical Trial Supplies Market Report 2024:
https://www.thebusinessresearchcompany.com/sample_request?id=12133&type=smp