Coronavirus (COVID-19) Current Therapy Market Size, Share, And Growth Rate Analysis 2023
The Business Research Company’s global market reports are now updated with the latest market sizing information for the year 2023 and forecasted to 2032
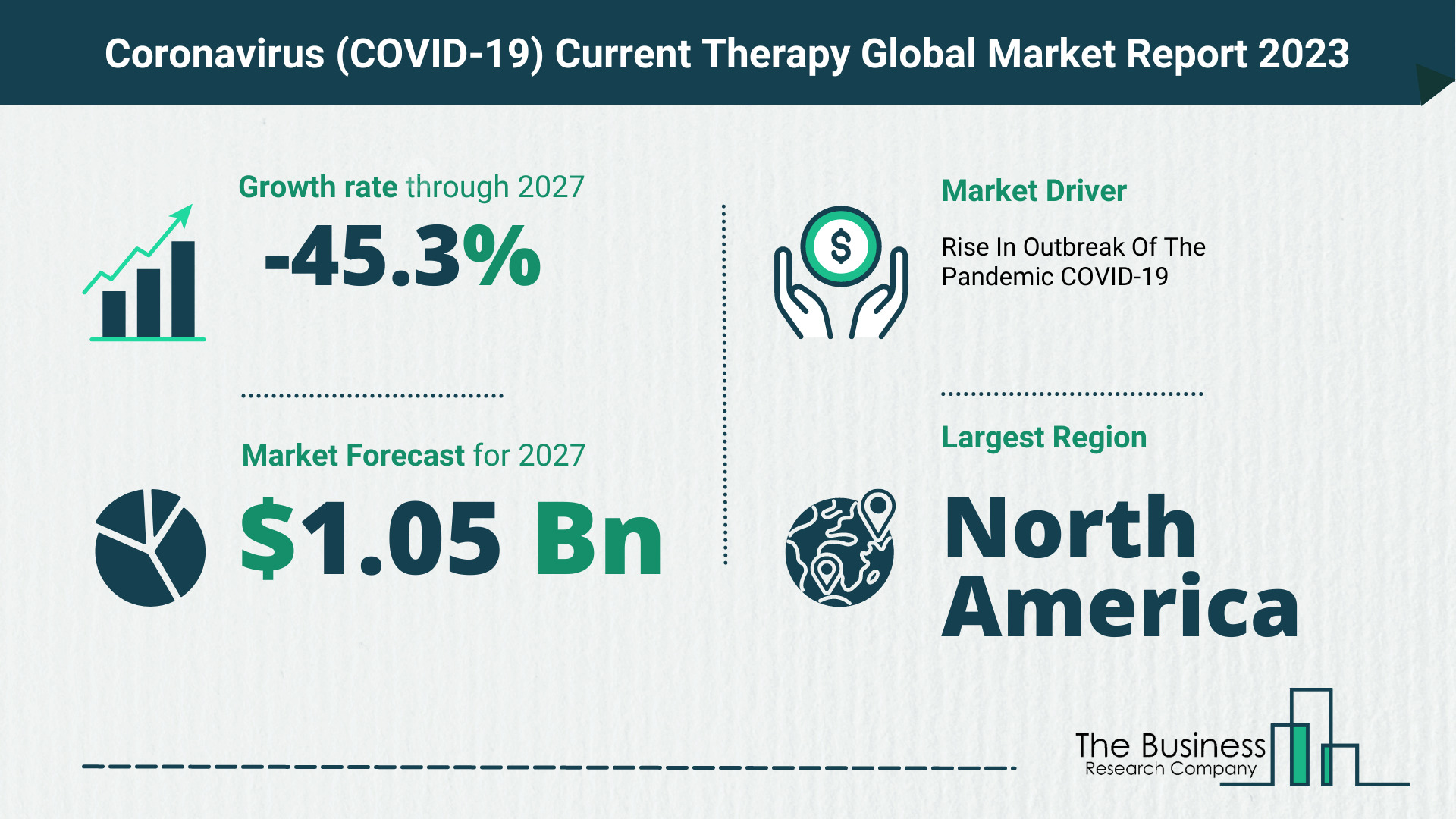
The Business Research Company’s coronavirus (COVID-19) current therapy market report forecasts the coronavirus (COVID-19) current therapy market size to grow to $1.05 Billion by 2027, with a CAGR (compound annual growth rate) of -45.3%.
Learn More On The Coronavirus (COVID-19) Current Therapy Market Report 2023 – https://www.thebusinessresearchcompany.com/report/coronavirus-current-therapy-global-market-report
Coronavirus (COVID-19) Current Therapy Market Size Forecast
The global coronavirus (COVID-19) current therapy market is expected to grow from $16.43 billion in 2022 to $11.73 billion in 2023 at a compound annual growth rate (CAGR) of -28.6%. The Russia-Ukraine war disrupted the chances of global economic recovery from the COVID-19 pandemic, at least in the short term. The war between these two countries has led to economic sanctions on multiple countries, a surge in commodity prices, and supply chain disruptions, causing inflation across goods and services and affecting many markets across the globe. The coronavirus (covid-19) current therapy market is expected to grow to $1.05 billion in 2027 at a CAGR of -45.3%.
North America held the largest coronavirus (COVID-19) current therapy market share, and Middle East was the fastest-growing region in 2022.
Key Coronavirus (COVID-19) Current Therapy Market Driver – Rise In Outbreak Of The Pandemic COVID-19
On March 11, 2020, the World Health Organization (WHO) declared the outbreak a global pandemic. According to the World Health Organization (WHO), there were 10,719,946 cases of COVID-19, including 517,337 deaths reported to the WHO on July 3, 2020, and this number is expected to grow soon. As there is no officially approved drug for COVID-19, the demand has risen significantly for repurposed drugs that are used for corona therapy. Countries across the world are facing shortages of drugs, and drug manufacturers are ramping up production to meet the global demand. The Coronavirus Treatment Acceleration Program (CTAP) is a special emergency programme initiated by the FDA for the development of potential COVID-19 therapies to be made available to patients as quickly as possible.
Request for A Sample Of The Global Coronavirus (COVID-19) Current Therapy Market Report:
https://www.thebusinessresearchcompany.com/sample.aspx?id=3247&type=smp
Key Coronavirus (COVID-19) Current Therapy Market Trend – Launching Convalescent Plasma Therapy
The blood plasma of patients who have recovered from a disease is called convalescent plasma (CP). Convalescent plasma therapy (CP) is a type of passive antibody therapy in which blood plasma is isolated from patients who have recovered from the disease of interest and administered to a patient with severe disease in order to suppress virulence and improve clinical symptoms.The blood plasma of recovered COVID-19 patients has antibodies to fight COVID-19 infection. According to guidance issued by the FDA, Convalescent Plasma Therapy is recommended as an investigational product during a public health emergency. By 30th April 2020, around 2,004 participating sites adhering to a single expanded access protocol by the US FDA had been registered, around 7,774 patients have enrolled, and 3,809 of them have undergone convalescent plasma transfusion. The experimental convalescent plasma therapy is likely to gain attention if enough data is supporting the results.
Coronavirus (COVID-19) Current Therapy Market Segment
1) By Drug Type: Remdesivir, Hydroxychloroquine, Ritonavir, Lopinavir, Interferon Beta, Other Drug Type
2) By Route Of Administration: Oral, Intravenous
3) By End User: Hospitals, Clinics, Research Institutes, and Other End Users
Coronavirus (COVID-19) Current Therapy Market Major Players and Strategies
Major players in the coronavirus (COVID-19) current therapy market are Moderna Therapeutics, Novavax, Bravovax, Ascletis Pharma, Altimmune, Clover Biopharmaceuticals, Inovio Pharmaceuticals, Inc., Biocryst Pharma, Gilead Sciences, and Regeneron Pharmaceuticals.
In March 2020, Sanofi and Regeneron Pharmaceuticals planned to initiate clinical trials of the rheumatoid arthritis drug Kevzara (sarilumab) for the treatment of COVID-19 symptoms. The US Food and Drug Administration (FDA) approved Kevzara to treat rheumatoid arthritis. The drug is part of an ongoing antibody partnership between Sanofi and Regeneron. Kevzara is a fully-human monoclonal antibody that inhibits the interleukin-6 (IL-6) pathway by binding and blocking the IL-6 receptor. IL-6 may play a role in driving the overactive inflammatory response in the lungs of patients who are severely or critically ill with COVID-19. Following a review by the Independent Data Monitoring Committee (IDMC) of all available Phase 2 and Phase 3 data, the trial will be immediately amended so that only critical patients continue to be enrolled to receive Kevzara 400 mg.
The Coronavirus (COVID-19) Current Therapy Global Market Report 2023 covers regional data on coronavirus (COVID-19) current therapy market size, coronavirus (COVID-19) current therapy market trends and drivers, opportunities, strategies, and coronavirus (COVID-19) current therapy market competitor analysis. The countries covered in the coronavirus (COVID-19) current therapy market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, the UK, and the USA, and the major seven regions are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, the Middle East, and Africa.
Coronavirus (COVID-19) current therapy refers to a drug created and used to treat mild to moderate COVID-19 in those who have a higher risk of experiencing significant illness as a result of COVID-19.
View More Reports Related To The Coronavirus (COVID-19) Current Therapy Market –
COVID-19 Detection Test Kits And Consumables Global Market Report 2023
COVID-19 Drug Associated APIs Global Market Report 2023
COVID-19 Rapid Test Kits Global Market Report 2023
Contact us at:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at [email protected]
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model