Electronic Trial Master File (eTMF) Systems Market 2025-2034: Key Highlights, Growth Dynamics, and Emerging Trends
Discover trends, market shifts, and competitive outlooks for the electronic trial master file (etmf) systems industry through 2025-2034 with The Business Research Company’s reliable data and in-depth research
#What Is the Current and Projected Market Size of the Electronic Trial Master File (eTMF) Systems Market Through 2034?#_x000D_
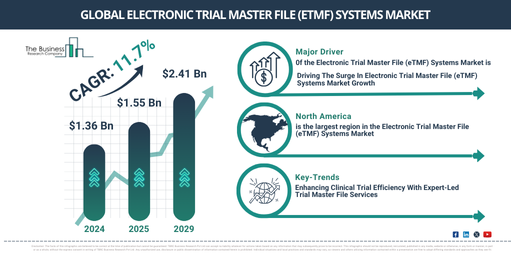
In recent times, the market size of electronic trial master file (eTMF) systems has experienced a significant increase. Its growth is projected to surge from $1.36 billion in 2024 to $1.55 billion in 2025, following a compound annual growth rate (CAGR) of 13.9%. The historical period of growth is largely attributed to factors like increasing needs for regulatory compliance, increased complexity in clinical trials, globalization of clinical trials, challenges associated with paper-based documentation, and an enhancing focus on data quality and integrity._x000D_
_x000D_
In the coming years, the electronic trial master file (eTMF) systems market is projected to experience substantial growth. It is forecasted to increase to a value of $2.41 billion by 2029, with a compound annual growth rate (CAGR) of 11.7%. Factors contributing to this predicted growth during the forecast period include the integration of electronic health records with eTMF, an increase in clinical trials, a move towards patient-centric trials, and a clear emphasis on real-time monitoring and reporting. Emerging trends to be noted in the forecast period include the rising demand for AI-enabled eTMF systems, heightened focus on data analytics and insights, increasing prominence of patient-centric trials, and the uptake of cloud-based eTMF solutions._x000D_
_x000D_
#Download a free sample to assess the report’s scope and structure:#_x000D_
https://www.thebusinessresearchcompany.com/sample.aspx?id=14098&type=smp_x000D_
_x000D_
#How Are Key Drivers in the Industry Acting as Catalysts for the Growth of the Electronic Trial Master File (eTMF) Systems Market?#_x000D_
The growth of the electronic trial master file (eTMF) systems market is predicted to be driven by the rising number of clinical trials. These trials, which meticulously examine and test the security, efficiency, and efficacy of medical interventions, treatments, or procedures on humans, aim to deliver trustworthy data and evidence to validate the safety and effectiveness of a new drug, therapy, or other medical intervention amongst a particular demographic of patients. The rise in clinical trials has a direct impact on the increasing adoption of electronic trial master files (eTMF) systems. These systems are crucial in managing documentation processes, confirming regulatory adherence, and improving collaboration with stakeholders in the face of growing volume and complexity of clinical research. According to a report by the Association of the British Pharmaceutical Industry released in November 2023, the total number of clinical trials has risen to 411 in 2022 from 394 in 2021. The UK’s industry clinical trials yearly recruitment figures increased by 5,366 participants to reach 42,088 in the 2022-23 period. As such, the increasing number of clinical trials is foreseen to fuel the electronic trial master file (eTMF) systems market’s progression._x000D_
_x000D_
#Which Segments in the Electronic Trial Master File (eTMF) Systems Offer the Most Growth?#_x000D_
The electronic trial master file (eTMF) systems market covered in this report is segmented –_x000D_
_x000D_
1) By Component: Services, Software_x000D_
2) By Delivery Mode: On-Premise, Cloud-Based_x000D_
3) By End-User: Pharmaceutical And Biotechnology Companies, Contract Research Organizations (CROs), Other End-Users_x000D_
_x000D_
Subsegments:_x000D_
1) By Services: Consulting Services, Implementation Services, Training And Support Services, Data Migration Services_x000D_
2) By Software: eTMF Management Software, Document Management Software, Cloud-Based eTMF Solutions, Compliance And Regulatory Software_x000D_
_x000D_
#Request customized data on this market:#_x000D_
https://www.thebusinessresearchcompany.com/customise?id=14098&type=smp_x000D_
_x000D_
#What Are the Fastest-Growing Geographies in the Electronic Trial Master File (eTMF) Systems Market?#_x000D_
North America was the largest region in the electronic trial master file (eTMF) systems market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the electronic trial master file (eTMF) systems market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa._x000D_
_x000D_
#What Are the Current Market Growth and Trends in the Electronic Trial Master File (eTMF) Systems Industry?#_x000D_
Leading corporations in the electronic trial master file (eTMF) systems market are emphasizing on producing advanced products like cloud-based eTMF to cater to the escalating demand. Cloud-based electronic trial master file can be described as a digital platform or system accessible through a cloud infrastructure, specifically built to handle and classify vital documents and data relevant to clinical trials in the life sciences and pharmaceutical sectors. For example, Montrium Inc., a software solutions firm based out of Canada that specializes in cloud-based platforms, introduced their cloud-based trial master file (TMF) services along with TMF maturity instructional training in September 2022. These services are tailored to assist clinical operations and TMF teams throughout every phase of clinical development. This extensive service portfolio is powered by TMF process experience and top-tier technology, effectively creating a level playing field for growing organizations by enabling them to reach the pinnacle of TMF excellence._x000D_
_x000D_
#View the full report here:#_x000D_
_x000D_
#What Are the Key Elements That Define the Electronic Trial Master File (eTMF) Systems Market?#_x000D_
An electronic trial master file (eTMF) is a trial master file in electronic or digital format, serving as a content management system for the pharmaceutical industry. The eTMF system manages, stores, tracks, and archives essential clinical study documents electronically. It is designed to ensure compliance with regulatory requirements, such as the FDA’s Title 21 CFR Part 11, and to provide a secure, centralized, and easily accessible repository for clinical trial documents._x000D_
_x000D_
#Purchase the full report and get a swift delivery:#_x000D_
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=14098_x000D_
_x000D_
#About The Business Research Company:#_x000D_
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game._x000D_
_x000D_
#Get in touch with us:#_x000D_
The Business Research Company: https://www.thebusinessresearchcompany.com/_x000D_
Americas +1 3156230293_x000D_
Asia +44 2071930708_x000D_
Europe +44 2071930708_x000D_
Email us at info@tbrc.info_x000D_
_x000D_
#Follow us on:#_x000D_
_x000D_
LinkedIn: https://in.linkedin.com/company/the-business-research-company_x000D_
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ_x000D_
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model