How Is The Companion Diagnostics Market Expected To Grow Through 2024-2033
The Business Research Company’s global market reports are now updated with the latest market sizing information for the year 2024 and forecasted to 2033
According to The Business Research Company’s Companion Diagnostics Global Market Report 2024, the companion diagnostics market is expected to show promising growth in the forecast period.
The companion diagnostics market has witnessed remarkable growth in recent years, with a substantial surge from $6.26 billion in 2023 to $7.52 billion in 2024, showcasing a robust Compound Annual Growth Rate (CAGR) of 20.1%. The historic growth can be attributed to various factors such as increased adoption of personalized medicine, the expansion of oncology and cancer therapies, heightened collaboration in pharmaceutical R&D, precision medicine initiatives, and advancements in biomarker discovery.
Anticipating the Future: Projected Expansion
Forecasted Growth and Catalysts
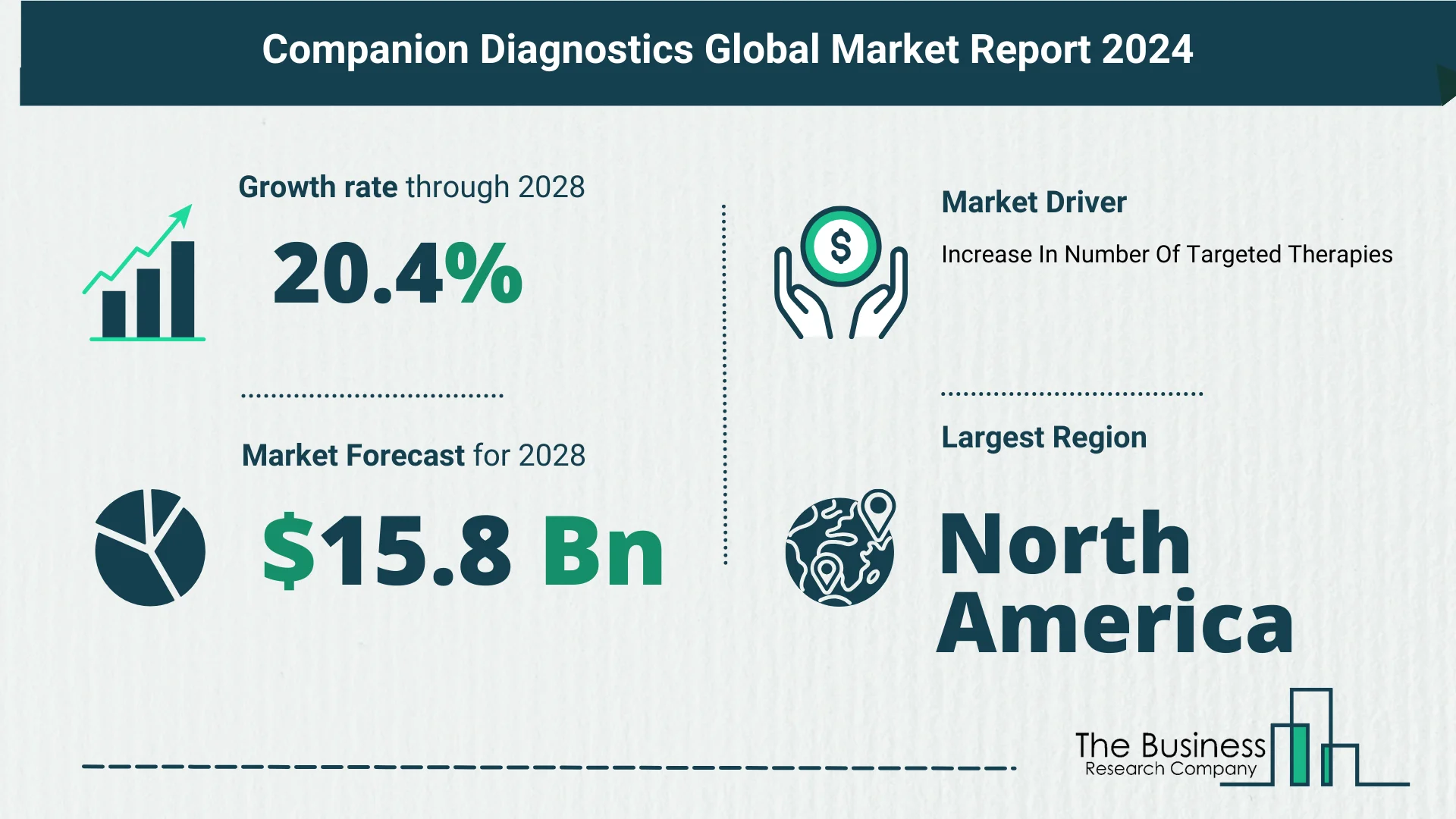
Looking ahead, the companion diagnostics market is poised for exponential growth, projected to reach $15.8 billion in 2028 with a CAGR of 20.4%. The forecasted expansion is fueled by factors including the development of targeted therapies, advancements in infectious disease diagnostics, increasing rare disease diagnostics, biomarker-based drug development, the rise of point-of-care testing, and applications of liquid biopsy. Major trends in the forecast period encompass immunotherapy and immunology advances, the integration of AI and machine learning in diagnostics, product innovations, the adoption of Industry 4.0, and strategic collaborations.
Targeted Therapies Propelling Market Growth
Understanding Targeted Therapies
The exponential growth of the companion diagnostics market is closely tied to the increasing prevalence of targeted therapies. Targeted therapy, a pharmacological approach focusing on specific characteristics of cancer cells, inhibits the growth and spread of diseases. With fewer adverse effects compared to chemotherapy, biological marker-based companion diagnostics are becoming integral for personalized cancer care. The European Society for Medical Oncology reported an estimated eligibility increase from 8.82% in previous years to 13.60% in 2020 for genome-targeted therapy, highlighting the growing prominence of these therapies.
- The increasing number of targeted therapies is a key driver for companion diagnostics market growth.
- Genome-targeted therapy eligibility rose from 8.82% to 13.60% in 2020.
Innovations Transforming Precision Medicine
Innovative Treatment Solutions
Major companies in the companion diagnostics market, including F Hoffmann-La Roche Ltd., Agilent Technologies Inc., Qiagen NV, and others, are at the forefront of developing groundbreaking solutions. The introduction of therascreen KRAS PCR Mutation Analysis exemplifies this innovation, offering a rapid and effective method to identify patients with non-small cell lung cancer. This companion diagnostic, launched by Laboratory Corporation of America Holdings, aids in identifying tumors with specific genetic mutations, ensuring patients receive the most effective and targeted treatments.
- Innovative treatment solutions, like therascreen KRAS PCR Mutation Analysis, contribute to market growth.
- The test aids in identifying patients with non-small cell lung cancer and specific genetic mutations.
Strategic Moves: Acquisitions Driving Market Dynamics
Agilent’s Acquisition of Resolution Bioscience
In April 2021, Agilent Technologies Inc. acquired Resolution Bioscience for $695 million, strengthening its position in NGS-based cancer diagnostics. This strategic move enhances Agilent’s capabilities in precision medicine, aligning with the demands of the rapidly expanding sector. Resolution Bioscience, known for its expertise in NGS technology and molecular biology, brings valuable assets to Agilent’s portfolio.
- Agilent Technologies’ acquisition of Resolution Bioscience enhances NGS-based cancer diagnostics capabilities.
- The acquisition facilitates access to technology in the rapidly growing precision medicine sector.
View More On The Companion Diagnostics Market Report 2024 – https://www.thebusinessresearchcompany.com/report/companion-diagnostics-global-market-report
Market Segmentation: Understanding the Landscape
Segmentation Overview
The companion diagnostics market is segmented based on:
- Product And Service: Assays, Kits And Reagents, Software And Services
- Technology: Polymerase Chain Reaction, Next-Generation Sequencing, In Situ Hybridization, Immunohistochemistry
- Indication: Lung Cancer, Breast Cancer, Colorectal Cancer, Leukemia, Melanoma
- End-User: Pharmaceutical And Biopharmaceutical Companies, Reference Laboratories, Contract Research Organizations
- Comprehensive segmentation provides insights into the diverse facets of the companion diagnostics market.
Regional Dominance: North America Leading the Way
North America’s Market Influence
In 2023, North America emerged as the largest region in the companion diagnostics market, underscoring its significant role in shaping market dynamics and trends.
- North America leads the companion diagnostics market, reflecting its influence in the sector.
Conclusion: Embracing a Dynamic Future
The companion diagnostics market is experiencing unparalleled growth, driven by factors such as the surge in targeted therapies, innovative treatment solutions, strategic acquisitions, and a robust focus on precision medicine. As the industry continues to evolve, staying attuned to emerging trends, technological advancements, and strategic collaborations will be essential for stakeholders looking to capitalize on the market’s exponential growth potential. The future of companion diagnostics promises transformative advancements, contributing to a more personalized and effective approach in the realm of diagnostics and therapeutics.
Request A Sample Of The Global Companion Diagnostics Market Report 2024:
https://www.thebusinessresearchcompany.com/sample_request?id=7038&type=smp