Global Electronic Trial Master File (eTMF) Systems Market Analysis: Estimated Market Size And Growth Rate
The Business Research Company’s global market reports are now updated with the latest market sizing information for the year 2024 and forecasted to 2033
Market Size and Growth Dynamics
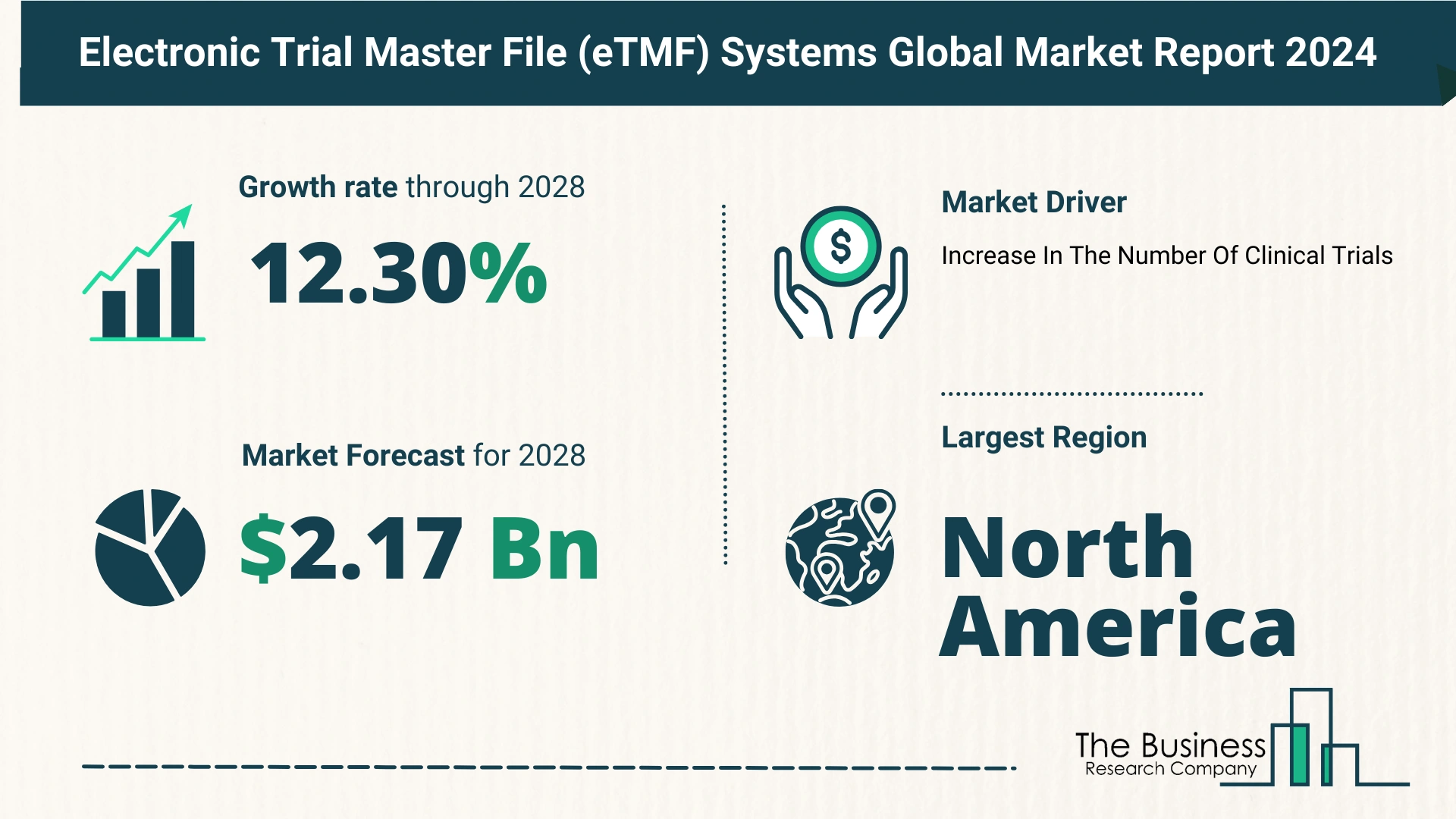
- The eTMF market grew from $1.19 billion in 2023 to $1.36 billion in 2024, with a CAGR of 14.0%.
- Projected to reach $2.17 billion by 2028, growing at a CAGR of 12.3%.
Factors Driving Market Growth
- Increasing regulatory compliance requirements.
- Rise in clinical trial complexity.
- Globalization of clinical trials.
- Challenges with paper-based documentation.
- Emphasis on data quality and integrity.
Driving The Surge In Electronic Trial Master File (ETMF) Systems Market Growth
The rise in clinical trials is a key driver for eTMF market growth, facilitating efficient management and documentation:
- Clinical trials provide essential data on medical interventions’ safety and efficacy.
- eTMF systems streamline documentation processes and ensure regulatory compliance.
- In the UK, clinical trials increased to 411 in 2022, supporting robust market expansion.
View More On The Electronic Trial Master File (eTMF) Systems Market Report 2024 – https://www.thebusinessresearchcompany.com/report/electronic-trial-master-file-etmf-systems-global-market-report
Major Players and Innovations in the Market
Leading companies shaping the eTMF systems market include:
- Veeva Systems
- Oracle
- TransPerfect
- Phlexglobal
- SureClinical Inc.
- Aurea, Inc.
- MasterControl, Inc.
- Clinevo Technologies
- Covance Inc.
- Ennov
- Care Lex
- ePharma Solutions
- Database Integrations, Inc.
- Aris Global LLC
- Mayo Foundation for Medical Education and Research
- arivis AG
- Wingspan Technology
- Dell EMC
- Paragon Solutions
- Freyr
- SAFE-BioPharma
- BIOVIA Corp.
Enhancing Clinical Trial Efficiency With Expert-Led Trial Master File Services
Companies are innovating with cloud-based eTMF solutions to meet industry demands:
- Montrium Inc. launched cloud-based TMF services in September 2022, enhancing clinical operations support.
- These offerings streamline TMF processes and ensure compliance throughout clinical development.
Strategic Acquisitions and Expansions
Veeva Systems’ acquisition of Veracity Logic underscores efforts to optimize clinical trial efficiency:
- The acquisition enhances eTMF capabilities, simplifying complex trial processes and accelerating timelines.
Market Segmentation
The eTMF systems market is segmented by:
- Component: Services, Software
- Delivery Mode: On-Premise, Cloud-Based
- End-User: Pharmaceutical and Biotechnology Companies, Contract Research Organizations (CROs), Other End-Users
Regional Insights
North America led the eTMF systems market in 2023, driven by stringent regulatory frameworks and advanced healthcare infrastructure. Asia-Pacific is poised for rapid growth, supported by increasing clinical trial activities and technological advancements.
In conclusion, the eTMF systems market is expanding rapidly, fueled by advancements in clinical trial management and regulatory compliance. Innovations in cloud-based solutions and strategic acquisitions are reshaping the industry landscape, enhancing operational efficiencies and supporting global clinical research initiatives. As the demand for real-time data analytics and patient-centric trials grows, the eTMF market continues to evolve, positioning key players to drive future innovations and advancements in healthcare.
Request A Sample Of The Global Electronic Trial Master File (eTMF) Systems Market Report 2024:
https://www.thebusinessresearchcompany.com/sample_request?id=14098&type=smp