Key Trends And Drivers In The Heart Defect Closure Device Market 2024
The Business Research Company’s market reports offer an in-depth analysis on the market’s growth potential, major drivers, key trends and more.
Market Overview
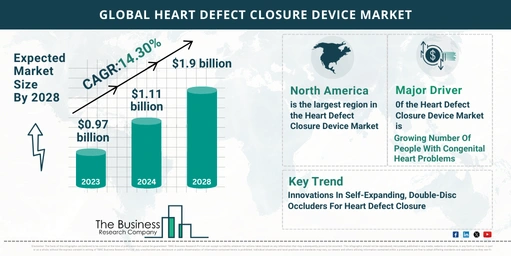
The heart defect closure device market has experienced significant growth recently and is projected to continue expanding.
- 2023: $0.97 billion
- 2024: $1.11 billion (CAGR of 14.2%)
- 2028: $1.90 billion (CAGR of 14.3%)
This growth is driven by several factors, including the increasing prevalence of congenital heart problems, an aging population, rising health awareness, and advancements in medical technology.
Key Drivers of Market Growth
- Rising Incidence of Congenital Heart Problems
- Structural defects in the heart present from birth are on the rise.
- Genetic factors, environmental influences, and maternal health issues contribute to the growing incidence.
- Heart defect closure devices provide minimally invasive treatments to correct these defects, improving cardiac function and reducing complications.
- Technological Advancements
- Improvements in catheterization techniques and diagnostic technologies are driving market growth.
- The rise in MRI procedures and the use of 3D imaging in heart defect closure are notable trends.
- Aging Population
- The prevalence of heart defects increases with age, impacting a significant portion of the elderly population.
Read More On The Heart Defect Closure Device Market Report 2024 – https://www.thebusinessresearchcompany.com/report/heart-defect-closure-device-global-market-report
Innovations in Heart Defect Closure Devices
- Self-Expanding, Double-Disc Occluders
- Companies are developing advanced occluders that enhance procedural safety and patient outcomes.
- Example: Abbott Laboratories’ Amplatzer Talisman PFO Occlusion System, launched in September 2022, uses a self-expanding, double-disc occluder to seal patent foramen ovale (PFO) with minimal invasiveness.
- Expansion of Product Portfolios
- Haemonetics Corporation’s acquisition of Cardiva Medical in March 2021 expands its range of vascular closure solutions, offering more options for heart defect treatments.
Major Market Players
The heart defect closure device market features several prominent companies:
- Abbott Laboratories
- Medtronic
- Koninklijke Philips N.V.
- Boston Scientific Corporation
- Terumo Corporation
- Edwards Lifesciences
- W L Gore and Associates
- BIOTRONIK SE & Co. KG
- Lepu Medical Technology
- Meril Life Sciences Pvt. Ltd.
- MicroPort Scientific Corporation
- AtriCure Inc.
- LifeTech Scientific Corporation
- Baylis Medical Company Inc.
Market Segmentation
- By Type
- Atrial Septal Defect (ASD) Closure Devices
- Left Atrial Appendage (LAA) Closure Devices
- Patent Foramen Ovale (PFO) Closure Devices
- Patent Ductus Arteriosus (PDA) Closure Devices
- Ventricular Septal Defect (VSD) Closure Devices
- By Material
- Nitinol-Based Devices
- Stainless Steel Devices
- Other Materials
- By Mode of Delivery
- Transcatheter Delivery
- Surgical Delivery
- Other Modes
- By End-User
- Hospitals
- Clinics
- Ambulatory Surgical Centers
- Other End-Users
Regional Insights
- North America: The largest market in 2023.
- Asia-Pacific: Expected to be the fastest-growing region in the forecast period.
Conclusion
The heart defect closure device market is poised for robust growth, fueled by technological innovations, increasing prevalence of heart defects, and advancements in diagnostic and treatment methods. The market’s future looks promising with continued development and adoption of advanced devices aimed at improving patient outcomes.
Request for A Sample Of The Global Heart Defect Closure Device Market Report:
https://www.thebusinessresearchcompany.com/sample_request?id=16074&type=smp