Top Trends Driving Innovation and Change in the HER2 Inhibitors Market: Increasing Focus On Developing Treatment Forher2-Low Breast Cancerto Gain A Competitive Edge
Discover trends, market shifts, and competitive outlooks for the her2 inhibitors industry through 2025-2034 with The Business Research Company’s reliable data and in-depth research
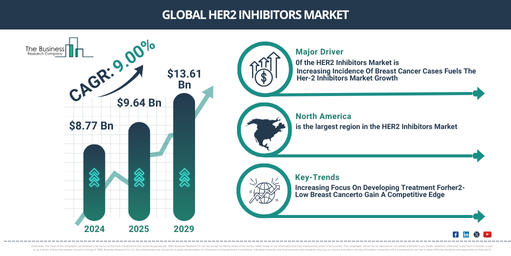
What is the Projected CAGR for the HER2 Inhibitors Market Size from 2025 to 2034?
In the past few years, the HER2 inhibitors market has experienced significant growth. It’s projected to expand from a value of $8.77 billion in 2024 to around $9.64 billion in 2025, showcasing a compound annual growth rate (CAGR) of 9.9%. This growth during the historic period is due in part to developments in biotechnology, the success of clinical trials, changes in treatment paradigms, increased investment in research and development, and an overall improvement in patient outcomes.
In the forthcoming years, robust growth is anticipated in the HER2 inhibitors market, with the market size reaching $13.61 billion in 2029, exhibiting a compound annual growth rate (CAGR) of 9.0%. The escalation during the expected span can be credited to factors such as patient-centric care models, regulation modifications, and approvals, drug resistance management, worldwide market penetration, broadened indications, and the implementation of precision oncology. Noteworthy trends in the forecast period comprise patient advocacy and awareness, clinical trials and research, early detection and diagnoses, precision medicine and biomarker testing, adjuvant and neo-adjuvant therapy.
Download a free sample to assess the report’s scope and structure:
https://www.thebusinessresearchcompany.com/sample.aspx?id=3396&type=smp
What External and Internal Drivers Are Contributing to the Growth of the HER2 Inhibitors Market?
The global escalation in breast cancer incidences is expected to foster the expansion of the HER-2 inhibitors market in the upcoming years. As reported by the American Cancer Society in January 2022, an estimated 1.9 million fresh cancer cases and 609,360 cancer-induced deaths are projected in the United States, approximating 1,670 deaths each day. Lung, prostate, bowel, and female breast cancer make up the four most prevalent cancer types globally, constituting 43% of all novel cancer diagnoses. Consequently, the surge in breast cancer rates worldwide is predicted to stimulate the demand for HER-2 inhibitors in the market in the approaching years.
What Segment Types Define the HER2 Inhibitors Market Structure?
The HER2 inhibitors market covered in this report is segmented –
1) By Treatment: Monotherapy, Combination Therapy
2) By Application: Squamous Cell Carcinoma, Adenocarcinoma, Large Cell Carcinoma, Breast Cancer, Other Applications
3) By End User: Hospitals, Clinics, Other End Users
Subsegments:
1) By Monotherapy: Single-Agent HER2 Inhibitors, Targeted HER2 Therapies
2) By Combination Therapy: HER2 Inhibitors Combined With Chemotherapy, HER2 Inhibitors Combined With Targeted Therapies, HER2 Inhibitors Combined With Hormonal Therapies
Request customized data on this market:
https://www.thebusinessresearchcompany.com/customise?id=3396&type=smp
Which Geographic Areas Hold the Strongest Growth Potential in the HER2 Inhibitors Market?
The countries covered in the HER2 inhibitors market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain
Which Emerging Trends that Are Influencing the HER2 Inhibitors Industry Evolution?
Leading firms in the HER2 inhibitor market are spearheading efforts to create treatments for HER2-low breast cancer, aiming to solidify their dominance in the marketplace. HER2-low breast cancer is a newly characterized subgroup of HER2-negative breast cancers, which are classified by the lack of overexpression or excessive replication of the HER2 protein. For instance, AstraZeneca PLC, a pharmaceutical and biotechnology corporation based in the UK, received approval from the U.S. Food and Drug Administration (FDA) in August 2022 for Enhertu (fam-trastuzumab deruxtecan-nxki). This makes it the premier HER2-targeted therapy for patients with HER2-low metastatic breast cancer. Administered via intravenous infusion, Enhertu is indicated for HER2-low (IHC 1+ or IHC 2+/ISH-) breast cancer in adults who have received prior metastatic chemotherapy or have had a relapse within half a year of finishing adjuvant chemotherapy.
View the full report here:
https://www.thebusinessresearchcompany.com/report/her2-inhibitor-global-market-report
What Is the Definition of the HER2 Inhibitors Market?
HER2 inhibitors refer to a group of drugs used to treat certain HER2-low breast malignancies as well as all stages of HER2-positive breast cancer, from early-stage to metastatic. Anti-HER2 drugs bind to the HER2 receptor proteins on the surface of breast cancer cells, and they function by preventing the HER2 receptors in HER2-positive breast cancer from receiving growth impulses.
Purchase the full report and get a swift delivery:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=3396
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Get in touch with us:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at info@tbrc.info
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model