$1204.56 Billion Forecast for In Vitro Diagnostics Market by 2029, Backed by Demand and Innovation
Discover trends, market shifts, and competitive outlooks for the in vitro diagnostics industry through 2025-2034 with The Business Research Company’s reliable data and in-depth research
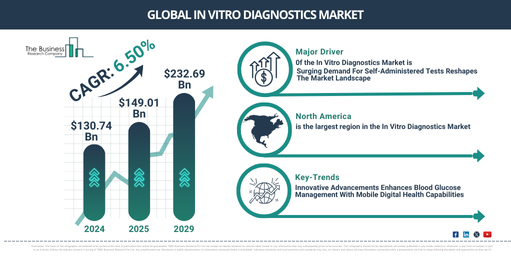
What Are the Key Projections for the CAGR of the In Vitro Diagnostics Market Size From 2025 to 2034?
The market size for in-vitro diagnostics has been experiencing swift expansion in the past few years. From $130.74 billion in 2024, it is projected to rise to $149.01 billion in 2025, boasting a compound annual growth rate (CAGR) of 14.0%. This significant growth in the historic period is due to the introduction of automated analyzers and molecular diagnostics, an increase in the elderly population, occurrence of disease outbreaks, healthcare legislations, and the rise of personalized medicine.
The market size for in-vitro diagnostics is anticipated to witness a significant surge in the coming years, expanding to a hefty $232.69 billion in 2029 at a Compound Annual Growth Rate (CAGR) of 11.8%. Factors contributing to this growth throughout the forecasted period include precision medicine, point-of-care testing, digital health and data analytics, and preparedness for pandemics. The forecasted period is also expected to see key trends such as technological developments, the integration of digital health, customized diagnostics, at-home testing kits, and molecular diagnostics.
Download a free sample to assess the report’s scope and structure:
https://www.thebusinessresearchcompany.com/sample.aspx?id=13065&type=smp
What Are the Core Growth Drivers Propelling the In Vitro Diagnostics Market Forward?
The surge in favorability for self-administered test kits is predicted to fuel the expansion of the in vitro diagnostics market. These test kits are medical or diagnostic tools, created for individuals to employ on their own, eliminating the necessity for healthcare experts or skilled workers to conduct the test at home. Such kits increase accessibility by allowing individuals to carry out diagnostic tests in the comfort of their homes, providing convenience and potentially amplifying the desire for testing, especially for conditions that were previously undiagnosed. For example, in April 2022, the Centers for Disease Control and Prevention, a US national public health institution, observed a marked escalation in the employment of at-home COVID-19 tests among American adults aged 18 and above from the Delta-dominant phase (August to December 2021) to the Omicron-dominant phase (December to March 2022). The utilization of at-home tests among participants showing COVID-19-like symptoms rose from 5.7% to 20.1%. Thus, the escalating favorability of self-administered test kits is poised to stimulate the expansion of the in vitro diagnostics market.
What Segment Types Define the In Vitro Diagnostics Market Structure?
The in vitro diagnostics market covered in this report is segmented –
1) By Type: Point-of-Care Diagnostics Devices And Equipment, Immunochemistry Diagnostic Devices And Equipment, Clinical Chemistry Diagnostics Devices And Equipment, Molecular Diagnostics Devices And Equipment, Microbiology Diagnostic Devices And Equipment, Hemostasis Diagnostic Devices And Equipment, Hematology Diagnostic Devices And Equipment, Immunohematology Diagnostic Devices And Equipment
2) By End User: Hospitals And Clinics, Diagnostic Laboratories, Other End Users
3) By Type of Expenditure: Public, Private
4) By Product: Instruments/Equipment, Disposables
Subsegments:
1) By Point-Of-Care Diagnostics Devices And Equipment: Blood Glucose Monitoring, Pregnancy And Fertility Testing, Infectious Disease Testing, Cardiac Markers Testing, Coagulation Testing
2) By Immunochemistry Diagnostic Devices And Equipment: Enzyme-Linked Immunosorbent Assay (ELISA), Radioimmunoassay (RIA), Western Blot, Immunofluorescence Assay, Chemiluminescence Immunoassay (CLIA)
3) By Clinical Chemistry Diagnostic Devices And Equipment: Blood Glucose Testing, Cholesterol Testing, Liver Function Testing, Kidney Function Testing, Electrolyte Testing
4) By Molecular Diagnostics Devices And Equipment: PCR (Polymerase Chain Reaction) Instruments, Next-Generation Sequencing (NGS) Instruments, Microarray Instruments, FISH (Fluorescence In Situ Hybridization) Devices
5) By Microbiology Diagnostic Devices And Equipment: Microbial Culture Systems, Automated Identification Systems, Antimicrobial Susceptibility Testing Systems, Blood Culture Systems
6) By Hemostasis Diagnostic Devices and Equipment: Coagulation Analyzers, Thromboelastography Systems, Platelet Function Analyzers, Clotting Factor Analyzers
7) By Hematology Diagnostic Devices and Equipment: Hematology Analyzers, Hemoglobin Testing Systems, Complete Blood Count (CBC) Devices, Blood Cell Counters
8) By Immunohematology Diagnostic Devices and Equipment: Blood Typing Systems, Crossmatching Systems, Antibody Screening Systems, Blood Bank Analyzers
Request customized data on this market:
https://www.thebusinessresearchcompany.com/customise?id=13065&type=smp
Which Geographic Areas Hold the Strongest Growth Potential in the In Vitro Diagnostics Market?
North America was the largest region in the global in-vitro diagnostics market in 2023. Asia-Pacific was the second largest region in the global in-vitro diagnostics market. The regions covered in the in-vitro diagnostics devices and equipment market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, and Africa.
What Are the Strategic Trends Steering the In Vitro Diagnostics Market Direction?
Key players in the in vitro diagnostics market are concentrating on the development of innovative products such as the Cobas pulse system, to enhance their market profitability. The Cobas pulse system is a high-quality blood glucose management tool with mobile digital health features to optimize patient treatment. For example, Roche Diagnostics, an American firm specializing in the creation of innovative products and services for disease prevention, diagnosis, monitoring, screening, and treatment, unveiled the Cobas pulse system in January 2022. This portable device comes with an automated glucose test strip reader, a camera, and a touchscreen for logging supplementary diagnostic information. Its easy-to-use interface facilitates seamless integration, offering convenient features that simplify operations, save time and boost workflow efficiency.
View the full report here:
https://www.thebusinessresearchcompany.com/report/in-vitro-diagnostics-global-market-report
What Is the Definition of the In Vitro Diagnostics Market?
In vitro diagnostics (IVD) refer to tests and procedures performed on biological samples, such as blood, tissue, or urine, taken from the human body to detect diseases, conditions, or infections. These tests are conducted outside of the body in a controlled environment, such as a laboratory, using specialized equipment and reagents. IVDs are crucial for diagnosing a wide range of health conditions, guiding treatment decisions, and monitoring patient health over time.
Purchase the full report and get a swift delivery:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=13065
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Get in touch with us:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at info@tbrc.info
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model