Key Trends And Drivers In The Influenza Diagnostic Market 2024
The Business Research Company’s market reports offer an in-depth analysis on the market’s growth potential, major drivers, key trends and more.
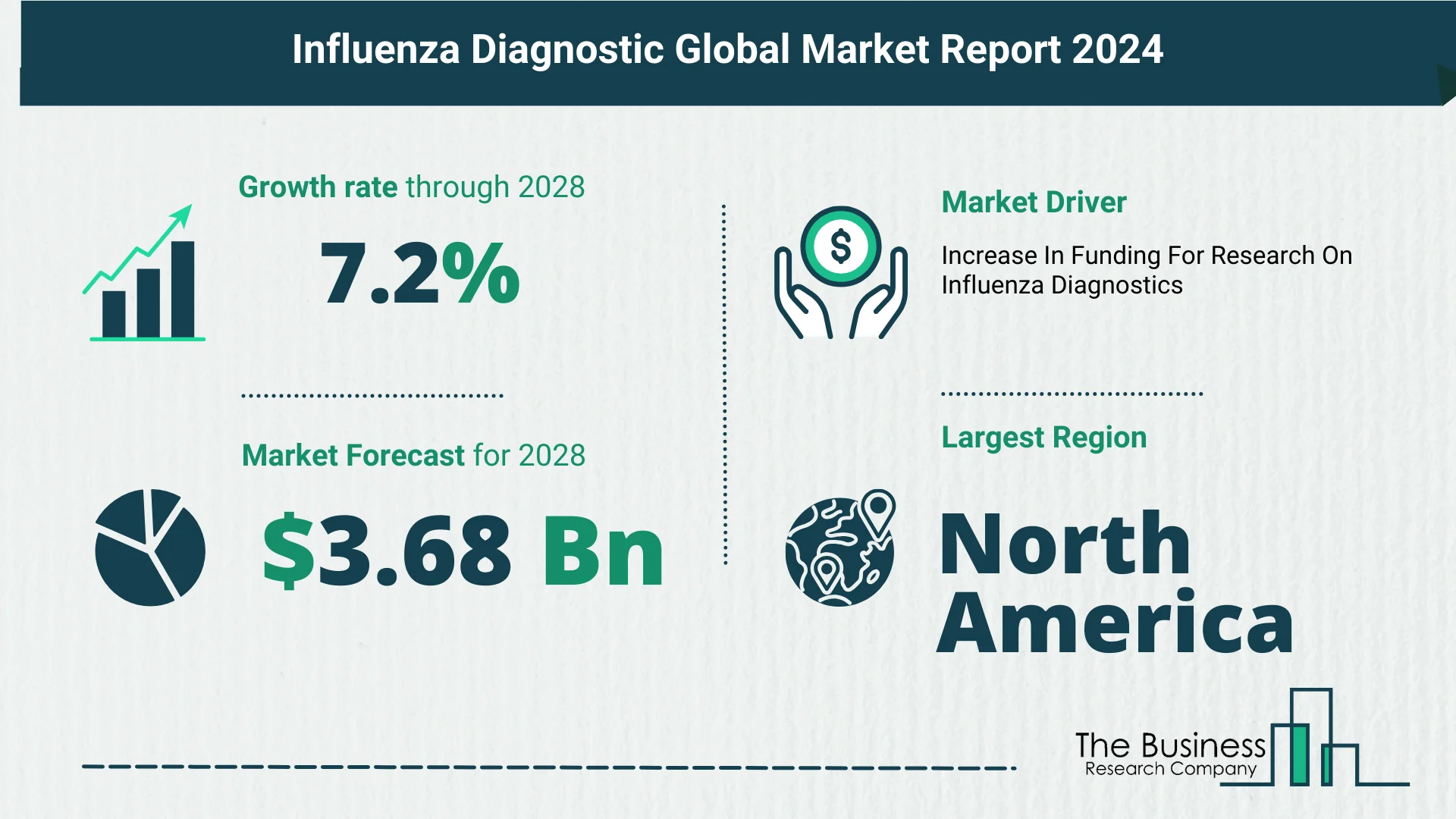
The influenza diagnostic market is poised for substantial growth in the coming years, with an expected surge to $3.68 billion in 2028, boasting a Compound Annual Growth Rate (CAGR) of 7.2%. This growth is attributed to factors such as the prevalence of seasonal influenza, the rise of point-of-care testing, the adoption of remote and home testing, and the integration of digital health solutions.
Rapid Diagnostic Tests: Propelling Market Growth
Understanding Diagnostic Tests
Diagnostic tests play a crucial role in confirming or ruling out various conditions and diseases. Rapid influenza diagnostic tests (RIDTs) are set to be a significant driver in the influenza diagnostic market. These tests can swiftly detect influenza viral antigens within 10-15 minutes, offering moderate sensitivity (50-70%). According to the Centers for Disease Control and Prevention (CDC), during September 2020 to May 2021 in the United States, 0.2% of respiratory specimens tested were positive for an influenza virus, highlighting the importance of rapid diagnostic tests.
Key Market Players
Major players in the influenza diagnostic market include F. Hoffmann-La Roche Ltd., Quidel Corporation, Thermo Fisher Scientific Inc., Abbott Laboratories, and Hologic Inc., among others. These companies are at the forefront of developing and providing innovative diagnostic solutions to meet the growing demand for influenza testing.
Simultaneous Detection: A Breakthrough in Diagnosis
Innovative Products Leading the Way
Major companies in the influenza diagnostic market are focusing on innovation, introducing breakthrough products like the Lucira COVID-19 & Flu Home Test. This at-home test kit allows simultaneous qualitative detection of influenza A and B (commonly known as the flu) and SARS-CoV-2 (the virus causing COVID-19). Pfizer Inc. received approval for this test in February 2023, highlighting the industry’s commitment to providing convenient and comprehensive diagnostic solutions.
Read More On The Influenza Diagnostic Market Report 2024 – https://www.thebusinessresearchcompany.com/report/influenza-diagnostic-global-market-report
Strength in Integration
The Lucira COVID-19 & Flu Home Test exemplifies the integration of diagnostic capabilities. With results available in approximately 30 minutes from self-collected nasal swab samples, this test showcases the industry’s dedication to offering rapid and reliable diagnostic solutions for both influenza and COVID-19.
Thermo Fisher’s Strategic Move: Mesa Biotech Acquisition
Strengthening Diagnostic Capabilities
In February 2021, Thermo Fisher Scientific Inc. made a strategic move by acquiring Mesa Biotech, Inc., an infectious disease diagnostic company. This acquisition aims to enhance Thermo Fisher’s manufacturing volume, achieve cost efficiencies, and expedite the delivery of diagnostics to the market on a larger scale. Such strategic partnerships and acquisitions contribute to the overall advancement and growth of the influenza diagnostic market.
Market Segmentation: Navigating the Landscape
Understanding Diagnostic Tests
The influenza diagnostic market is intricately segmented based on traditional diagnostic tests and molecular diagnostic tests. Traditional diagnostic tests include Rapid Influenza Diagnostic Tests (RIDT), Viral Culture, DFA, Serological assays, and other traditional diagnostic tests. On the other hand, molecular diagnostic tests encompass Reverse Transcription Polymerase Chain Reaction (RT-PCR), Isothermal Nucleic Acid Amplification Tests (INAAT), Loop-Mediated Isothermal Based Amplification Assays, and other molecular diagnostic tests.
End User Perspectives
End-user segments in the influenza diagnostic market include Hospitals and Clinical Laboratories, Diagnostic Reference Laboratories, Academic/Research Institutes, and Other End Users. Understanding these segments is crucial for comprehending the diverse applications and demands within the influenza diagnostic landscape.
Regional Leadership: North America Takes the Lead
Dominance in 2023
In 2023, North America emerged as the largest region in the influenza diagnostic market. This regional dominance can be attributed to a robust healthcare infrastructure, technological advancements, and a proactive approach in adopting diagnostic solutions. As the market continues to evolve, North America remains a key player shaping the trajectory of influenza diagnostics.
In conclusion, the influenza diagnostic market is on the cusp of significant growth, driven by the rise of rapid diagnostic tests, simultaneous detection solutions, strategic acquisitions, and a comprehensive understanding of market segmentation. With advancements in diagnostic technologies, the industry is poised to play a pivotal role in efficiently managing influenza and other respiratory illnesses in the years to come.
Request for A Sample Of The Global Influenza Diagnostic Market Report:
https://www.thebusinessresearchcompany.com/sample_request?id=3237&type=smp