Lucentis (Ranibizumab) Market Outlook 2025–2034: Identifying Growth Drivers, Technology Trends, and Policy Impact
Discover trends, market shifts, and competitive outlooks for the lucentis (ranibizumab) global market report 2025 industry through 2025-2034 with The Business Research Company’s reliable data and in-depth research
What Are the Key Milestones in the Lucentis (Ranibizumab) Market’s Growth Trajectory From 2025 To 2034?
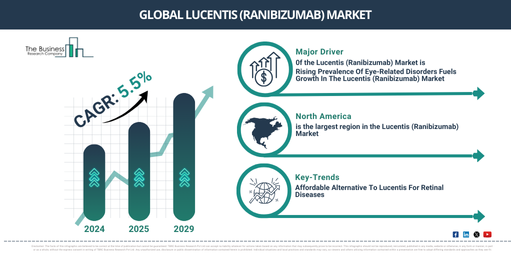
The market size of Lucentis (Ranibizumab) has witnessed significant expansion in prior years. Forecasts indicate expansion from $3,538.57 million in 2024 to $3,741.97 million in 2025, demonstrating a Compound Annual Growth Rate (CAGR) of 5.7%. This growth during the historic period can be traced back to factors like the increasing instances of age-related macular degeneration (AMD), the approval and reimbursement provisions by regulatory authorities, effective and favorable clinical outcomes, robust brand recognition and usage by physicians, along with the rise of biosimilars as well as competition on pricing.
Significant growth is anticipated in the ranibizumab (Lucentis) market size over the coming years, with expectations for it to reach $4,633.35 million by 2029, demonstrating a compound annual growth rate (CAGR) of 5.5%. This projected expansion can be credited to several factors. These include an escalating prevalence of diabetic retinopathy (DR) and diabetic macular edema (DME), enhancements in treatment methodologies and combination therapies, an aging demographic coupled with increasing instances of retinal disorders, the broadening market reach in emergent economies, and ongoing clinical investigations and potential novel indications. Trends predicted for future include a shift toward prolonged-interval dosing and maintenance treatment, rising inclination for biosimilars and alternative treatments, an uptick in tailored treatment methods for retinal diseases, amplified demand in emerging marketplaces owing to improved healthcare accessibility, and the embedding of digital health technologies in treatment monitoring.
Download a free sample to assess the report’s scope and structure:
https://www.thebusinessresearchcompany.com/sample.aspx?id=19907&type=smp
What are the Fundamental Drivers and Innovations Shaping the Lucentis (Ranibizumab) Market?
A surge in eye-related disorders is anticipated to fuel the expansion of the Lucentis (ranibizumab) market in the future. These disorders, which can impair the eyes’ proper functioning, include a broad spectrum of diseases and conditions. The escalation in cases of eye-related disorders can be attributed to factors such as aging, hereditary predisposition, unhealthy lifestyle choices including smoking and poor diet, as well as environmental factors like long-term sun exposure or usage of digital screens. Lucentis works to counteract the vascular endothelial growth factor (VEGF), a protein that causes abnormal blood vessel growth and leakage in the retina, leading to conditions such as age-related macular degeneration (AMD) and diabetic macular edema (DME). For example, the National Eye Institute, a US government agency, projected that by 2030, 2.2 million Americans would be blind. Therefore, the surging cases of eye-related disorders are augmenting the growth of the Lucentis (ranibizumab) market. The accelerated pace of healthcare spending is also predicted to stimulate the growth of the Lucentis (ranibizumab) market. Health care spending encompasses the total financial resources dedicated to healthcare services and products by individuals, governments, private insurance companies, and other entities. It’s growth is associated with an aging population, medical technology advancements, increased chronic disease prevalence, and a higher demand for healthcare services. This spending facilitates the increased accessibility and availability of Lucentis (ranibizumab) through its distribution, reimbursement, and coverage by healthcare systems, which subsequently enhances patient outcomes and eases the lasting impact of vision loss. For example, the Office for National Statistics, a UK government department, reported in May 2024 that there was a 5.6% rise in the country’s healthcare spending between 2022 and 2023, compared to a 0.9% growth in 2022. The expenditure on healthcare in the UK was approximately $317.63 billion (£292 billion) in 2023. Hence, as healthcare spending increases, so does the growth of the Lucentis (ranibizumab) market.
How Is the Lucentis (Ranibizumab) Market Segmented?
The lucentis (ranibizumab)market covered in this report is segmented –
1) By Type: Single-use Prefilled Syringe; Single-use Glass Vial
2) By Application: Age-related Macular Degeneration; Diabetic Retinopathy; Retinal Vein Occlusion; Myopic Choroidal Neovascularization; Uveitis
3) By End-User: Hospitals; Clinics; Ambulatory Surgical Centers; Other End-Users
Request customized data on this market:
https://www.thebusinessresearchcompany.com/customise?id=19907&type=smp
Which Regions Are Driving the Next Phase of the Lucentis (Ranibizumab) Market Growth?
North America was the largest region in the lucentis (ranibizumab) market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the lucentis (ranibizumab) market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
What Key Trends Are Shaping the Future of the Lucentis (Ranibizumab) Market?
The significant market development for Lucentis (ranibizumab) revolves around the creation of biosimilar alternatives that offer a cost-effective solution compared to the original branded product. Biosimilars are biological medications that closely resemble an already authorized reference biologic in structure, efficacy, and safety, presenting no clinically significant discrepancies. For example, In April 2024, the biosimilar rendition of Lucentis (ranibizumab), labeled FYB201, was released in Canada and Switzerland, through the efforts of Formycon AG, a biotech company based in Germany, and Bioeq AG, a Swiss biotech firm. The respective health agencies of both countries, Health Canada and Swiss Medic, granted marketing authorizations for the biosimilar, renaming it as Ranopto and Ranivisio correspondingly. The affirmation and ensuing introduction of FYB201 are meant to provide a cost-effective therapeutic option for patients dealing with retinal disorders. Its functioning mechanism includes blocking vascular endothelial growth factor A (VEGF-A), a protein that instigates abnormal blood vessel development in the retina, resulting in swelling, leakage, and potential vision damage.
View the full report here:
https://www.thebusinessresearchcompany.com/report/lucentis-ranibizumab-global-market-report
How Is the Lucentis (Ranibizumab) Market Defined and What Are Its Core Parameters?
Lucentis (ranibizumab) is a prescription medication used to treat various eye disorders, those involving abnormal blood vessel growth in the retina. It is a recombinant monoclonal antibody fragment designed to inhibit vascular endothelial growth factor (VEGF), a protein that promotes the formation of abnormal blood vessels.
Purchase the full report and get a swift delivery:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=19907
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Get in touch with us:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at info@tbrc.info
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model