Medical Device Affairs Outsourcing Market 2025-2034: Key Highlights, Growth Dynamics, and Emerging Trends
Discover trends, market shifts, and competitive outlooks for the medical device affairs outsourcing industry through 2025-2034 with The Business Research Company’s reliable data and in-depth research
#What Are the Key Milestones in the Medical Device Affairs Outsourcing Market’s Growth Trajectory From 2025 To 2034?#_x000D_
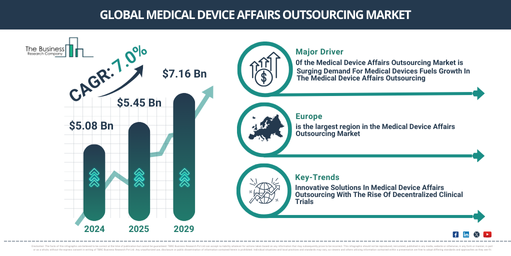
The market size for medical device affairs outsourcing has seen considerable growth in the past few years. This growth trajectory is set to continue, moving from a value of $5.08 billion in 2024 to $5.45 billion in 2025 with a compound annual growth rate (CAGR) of 7.3%. Factors contributing to this historical growth include the ever-tightening and developing regulatory demands, an escalating need for specialised proficiency in regulatory affairs, and an increased concentration on core competencies._x000D_
_x000D_
Expectations are high for robust expansion of the medical device affairs outsourcing market in the coming years. The market is forecasted to soar to $7.16 billion by 2029, recording a compound annual growth rate (CAGR) of 7.0%. Factors contributing to this growth through the forecast period include an increasing demand for medical devices, a growing necessity for regulatory approvals and expertise, and increasing healthcare expenditures. Key trending developments during the forecast period will likely be an upswing in the use of digital health technologies, a trend toward personalization in medicine and medical devices, an integrated application of AI and machine learning in medical devices, and a heightening emphasis on environmental sustainability._x000D_
_x000D_
#Download a free sample to assess the report’s scope and structure:#_x000D_
https://www.thebusinessresearchcompany.com/sample.aspx?id=19571&type=smp_x000D_
_x000D_
#How Are Key Drivers in the Industry Acting as Catalysts for the Growth of the Medical Device Affairs Outsourcing Market?#_x000D_
The growing need for medical devices is anticipated to accelerate the development of the medical device affairs outsourcing market in the future. Medical devices, defined as instruments, machinery, apparatuses or implants utilized for the diagnosis, prevention or treatment of medical conditions, are seeing increased demand due to enhanced patient results, elevated efficacy in treatment, and the proliferation of healthcare facilities. The practice of outsourcing medical device affairs provides a way for companies to streamline operations, fortify compliance and better market results, thereby freeing up internal resources to focus on innovation and growth, supported by external expertise for key functions. For example, The Medicines and Healthcare products Regulatory Agency (MHRA), a government agency based in the UK, saw a growth in the total number of medical products registered with the MHRA from approximately 2.25 million in 2022 to approximately 3.25 million by April 2024. Consequently, this rising demand for medical devices underpins the continuing expansion of the medical device affairs outsourcing market._x000D_
_x000D_
#Which Segments in the Medical Device Affairs Outsourcing Offer the Most Growth?#_x000D_
The medical device affairs outsourcingmarket covered in this report is segmented –_x000D_
_x000D_
1) By Service: Regulatory Writing and Submissions, Regulatory Registration Services, Regulatory Consulting, Other Services_x000D_
2) By Software: Cloud Based Software, On Premise Software_x000D_
3) By End User: Pharmaceutical Companies, Medical Technology Companies, Other End Users_x000D_
_x000D_
Subsegments:_x000D_
1) By Regulatory Writing And Submissions: Clinical Trial Applications (CTAs), Investigational New Drug Applications (INDs), New Drug Applications (NDAs), Summary And Reports For Regulatory Agencies_x000D_
2) By Regulatory Registration Services: Device Registration, Market Authorization Applications (MAAs), Product Listing And Establishment Registration_x000D_
3) By Regulatory Consulting: Strategic Regulatory Planning, Regulatory Compliance Audits, Labeling And Advertising Compliance_x000D_
4) By Other Services: Post-Market Surveillance, Training And Education, Quality Management System (QMS) Support, Risk Management And Assessment_x000D_
_x000D_
#Request customized data on this market:#_x000D_
https://www.thebusinessresearchcompany.com/customise?id=19571&type=smp_x000D_
_x000D_
#What Are the Fastest-Growing Geographies in the Medical Device Affairs Outsourcing Market?#_x000D_
Europe was the largest region in the medical device affairs outsourcing market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the medical device affairs outsourcing market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa._x000D_
_x000D_
#What Are the Key Market Trends in the Medical Device Affairs Outsourcing Market Over the Coming Years?#_x000D_
Prominent entities functioning in the medical device affairs outsourcing market are endeavoring to boost decentralized clinical trials in order to improve clinical research’s efficiency and flexibility, optimize patient enrollment and maintenance, and simplify the data aggregation and examination processes. A decentralized clinical trial (DCT) is a kind of clinical trial format that backs the medical device segment by offering unique services that amplify efficiency, adherence, and productivity of clinical trials – a fundamental step in introducing new medical devices into the market. For example, in March 2023, Syneos Health, an American organization offering contract research and commercial services to the pharmaceutical and biotech sectors, initiated a Decentralized Clinical Trial (DCT) Site Network. This aimed at augmenting the acceptance and quality of decentralized clinical trials’ pledge to further decentralized solutions in clinical trials, thereby enhancing patient involvement and the results of the trial. This amalgamation aids the gathering of strong evidence and real-world understandings, vital for successful commercialization strategies._x000D_
_x000D_
#View the full report here:#_x000D_
_x000D_
#What Are the Key Elements That Define the Medical Device Affairs Outsourcing Market?#_x000D_
Medical device affairs outsourcing refers to the practice of hiring external firms or experts to manage regulatory compliance and quality assurance tasks related to medical devices. This outsourcing strategy is increasingly adopted by medical device companies to navigate the complex and evolving regulatory landscape, ensuring adherence to various international standards and regulations._x000D_
_x000D_
#Purchase the full report and get a swift delivery:#_x000D_
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=19571_x000D_
_x000D_
#About The Business Research Company:#_x000D_
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game._x000D_
_x000D_
#Get in touch with us:#_x000D_
The Business Research Company: https://www.thebusinessresearchcompany.com/_x000D_
Americas +1 3156230293_x000D_
Asia +44 2071930708_x000D_
Europe +44 2071930708_x000D_
Email us at info@tbrc.info_x000D_
_x000D_
#Follow us on:#_x000D_
_x000D_
LinkedIn: https://in.linkedin.com/company/the-business-research-company_x000D_
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ_x000D_
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model