What Is The Forecast Growth Rate For The Medical Devices Vigilance Market?
The Business Research Company’s market reports offer an in-depth analysis on the market’s growth potential, major drivers, key trends and more.
The medical device vigilance market has shown robust growth in recent years, driven by various factors contributing to its expansion.
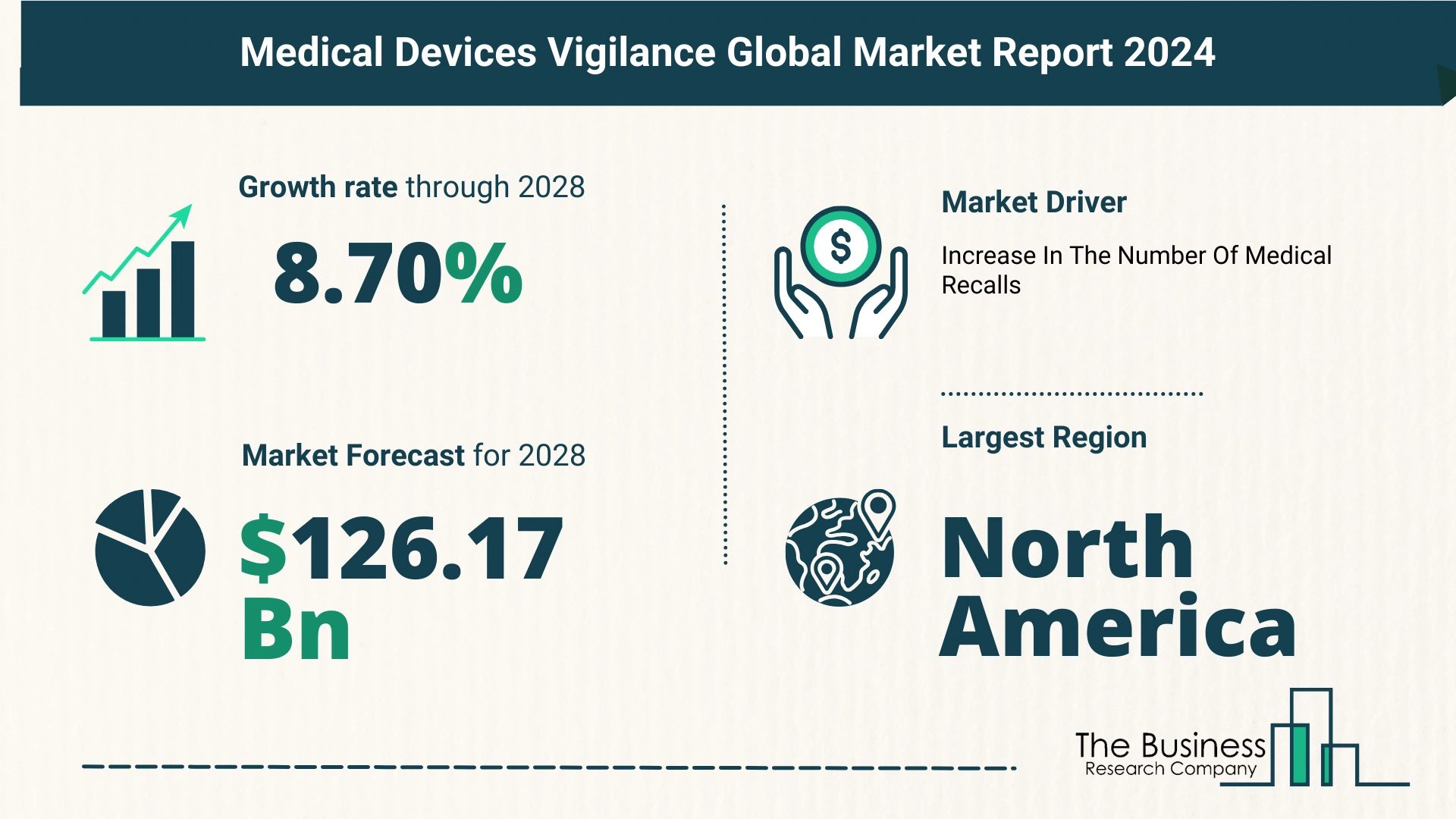
- Market Size Expansion: From $82.52 billion in 2023 to $90.26 billion in 2024, with a CAGR of 9.4%.
- Historic Growth Drivers:
- Increasing awareness of medical device vigilance benefits.
- Growing pressure on manufacturers for compliance.
- Government initiatives enhancing vigilance protocols.
- Rise in medical device usage among physicians and patients.
Projected Growth and Key Drivers
The market is set to continue its strong growth trajectory, reaching $126.17 billion by 2028, with an anticipated CAGR of 8.7%.
- Forecast Period Drivers:
- Introduction of recall systems for medical devices.
- Expansion of post-market surveillance programs.
- Rising incidents of adverse events reported.
- Increasing demand for therapeutic and surgical procedures.
- Stringent patient safety regulations.
- Emerging Trends:
- Technological advancements in medical device safety.
- Adoption of IMDRF frameworks for global safety data exchange.
- Growth in remote patient monitoring devices.
- Utilization of 3D printing for personalized medical devices.
Read More On The Medical Devices Vigilance Market Report 2024 – https://www.thebusinessresearchcompany.com/report/medical-devices-vigilance-global-market-report
Surge in Medical Device Recalls Drives Market Expansion
The escalation in medical device recalls plays a pivotal role in augmenting the medical device vigilance market.
- Definition and Impact: Regulatory actions to mitigate risks associated with non-compliant or unsafe medical products.
- Recent Trends: FDA reported a significant rise in recalls, emphasizing the need for robust vigilance practices.
- Impact: Increased vigilance requirements boost market demand for monitoring and reporting systems.
Leading Players and Innovations
Prominent companies are leveraging innovations to enhance medical device quality management and compliance.
- Greenlight Guru: Pioneering QMS software to streamline regulatory compliance and accelerate product development.
- GE Healthcare: Strengthening digital imaging capabilities through strategic acquisitions like MIM Software Inc.
Market Segmentation and Regional Insights
The market segmentation reflects diverse delivery modes, applications, and end-users.
- Delivery Mode: On-Demand and On-Premise solutions cater to varied operational needs.
- Application: Therapeutics, Diagnostics, Surgical, Research, and other specialized applications.
- End-User: OEMs, CROs, and BPO firms drive market engagement.
- Regional Dynamics:
- North America: Largest market share in 2023.
- Asia-Pacific: Expected to witness the fastest growth, driven by technological adoption and regulatory advancements.
Conclusion
The medical device vigilance market continues to evolve with increasing regulatory scrutiny and technological advancements. As industry players innovate and expand their capabilities, the market’s growth prospects remain promising, driven by heightened awareness, regulatory frameworks, and technological integration.
In summary, the future of medical device vigilance lies in proactive safety measures, technological innovations, and global regulatory alignment, ensuring patient safety and product efficacy in a dynamic healthcare landscape.
Request for A Sample Of The Global Medical Devices Vigilance Market Report:
https://www.thebusinessresearchcompany.com/sample_request?id=14137&type=smp