Unlocking Opportunities in the Medical Devices Vigilance Market: Key Trends, Market Growth, and Forecast Insights
2025 Market Reports Update: Market Size Forecasts to 2034, Key Trends, Leading Players, and Top Regions – Get Ahead of the Competition Today!
What Drivers Are Shaping the Growth and Development of theMedical Devices Vigilance Market?
The medical device oversight market is projected to expand, driven by the rising number of medical product withdrawals. A medical recall is a corrective measure taken by manufacturers, regulatory bodies, or distributors to eliminate or correct medical goods that pose a public health threat or fail to comply with regulatory criteria. Medical device vigilance involves the continual surveillance of medical devices on the market to detect adverse occurrences, malfunctions, or safety issues. These findings are conveyed to regulatory agencies, which may instigate further inquiries and possibly initiate a product recall if necessary. For example, in December 2022, a significant increase in medical device recalls was noted by the US-based federal institution, the Food and Drug Administration (FDA), with the number of recalls in 2022 reaching 442, a nearly 10% increment from the 331 recalls in 2021. Consequently, the mounting tally of medical recalls is fueling the growth of the medical device vigilance market.
Get Your Free Sample Report Now – Explore Exclusive Market Insights:
https://www.thebusinessresearchcompany.com/sample.aspx?id=14137&type=smp
#What Growth Opportunities Will Drive the Medical Devices Vigilance Market’s CAGR Through 2034?
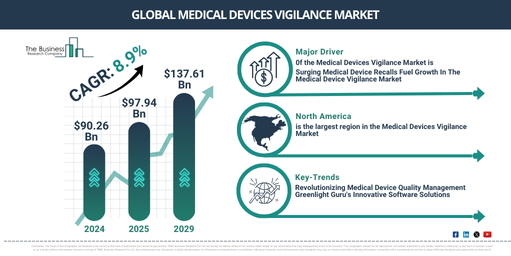
The market for medical-device vigilance has seen robust growth over the years, and it’s projected to expand further from its value of $90.26 billion in 2024 to $97.94 billion in 2025 with the compound annual growth rate (CAGR) rising at 8.5%. The earlier growth pattern was driven by several factors, including heightened awareness about medical device vigilance, understanding among physicians and patients about its advantages, mounting pressure on the manufacturers of these medical devices, heightened government initiatives, and increased usage of medical devices.
Anticipated to experience robust growth in the coming years, the vigilance market for medical devices is set to increase to a whopping $137.61 billion in 2029, with a compound annual growth rate (CAGR) of 8.9%. This projected expansion in the given timeframe can be credited to several factors such as the proliferation of recall systems for medical devices, the rise in post market surveillance programs for medical devices, escalating reports of adverse events, growing need for therapeutic and surgical procedures, and the escalating complexity of regulations pertaining to patient safety. The important forthcoming trends include technological innovations, the framework of the international medical device regulators forum (IMDRF), improvement of safety information exchange internationally, monitoring devices for remote patients, and the advent of 3D printing and personalized devices.
You can Directly Purchase the Report Here:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=14137
Which Cutting-Edge Market Trends Are Expected to Drive theMedical Devices Vigilance Market’s Growth?
Leading businesses within the medical device vigilance market are creating novel products like quality management software to enhance user satisfaction. Quality management software (QMS) is a structured system that records processes, procedures, and duties necessary to meet quality policies and goals. For example, Greenlight Guru, an American company offering systems and software to medical device companies, rolled out its QMS software in December 2022. This software aims to assist medical device businesses in modernising their operations, reducing clinical testing time, and hastening the market introduction of safer products. The software from Greenlight Guru conforms to FDA and ISO best practices, helping companies keep up with the constantly evolving standards. Additionally, Greenlight Guru provides training and certification to foster career growth in product development, quality, and regulatory assurance.
What Are the Leading Market Players Impacting theMedical Devices Vigilance Market’s Growth Trend?
Major companies operating in the medical devices vigilance market are Johnson & Johnson, Intel Corporation, Oracle Corporation, Medtronic, Siemens Healthineers, RELX Group plc, MasterControl Inc., Laerdal Medical, Numerix, Smithers, Omnify Software Inc., Freyr, Sparta Systems, MDI Consultants Inc., Greenlight Guru, Jama Software, Sarjen Systems Pvt. Ltd, Arena Solutions Inc., Xybion Corporation, ZEINCRO Group, Extedo Gmbh, AssurX Inc., AB-Cube, Panacea Pharma Projects Limited, Qvigilance, General Electric (GE) Healthcare, Philips Healthcare, Baxter International, Stryker Corporation, Boston Scientific
Order Your Report Now For A Swift Delivery:
https://www.thebusinessresearchcompany.com/report/medical-devices-vigilance-global-market-report
How Are the Key Segments of the Medical Devices Vigilance Market Driving Opportunities and Innovations?
The medical devices vigilance market covered in this report is segmented –
1) By Delivery Mode: On-Demand, On-Premise
2) By Application: Therapeutics, Diagnostics, Surgical, Research, Other Applications
3) By End-User: Original Equipment Manufacturers (OEMs), Clinical Research Organizations (CROs), Business Process Outsourcing (BPO) Firms
Subsegments:
1) By On-Demand: Cloud-Based Solutions, Subscription Services, Remote Monitoring And Reporting
2) By On-Premise: In-House Software Solutions, Local Server Deployments, Customized System Implementations
Gain Exclusive Market Insights—Customize Your Research Report Today for Fast Delivery!
https://www.thebusinessresearchcompany.com/customise?id=14137&type=smp
What Regions Are At the Forefront of #What Drivers Are Shaping the Growth and Development of theMedical Devices Vigilance Market?# Market Expansion?
North America was the largest region in the medical devices vigilance market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the medical devices vigilance market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
Browse Through More Reports Similar to the Global Medical Devices Vigilance Market 2025, By The Business Research Company:
Temperature Monitoring Devices Global Market Report 2024
Respiratory Monitoring Devices Global Market Report 2024
Respiratory Devices And Equipment (Therapeutic And Diagnostic) Global Market Report 2024
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Contact us at:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at [email protected]
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
Found this article helpful? Share it on: