Neupogen (filgrastim) Market 2025-2034: Key Highlights, Growth Dynamics, and Emerging Trends
Discover trends, market shifts, and competitive outlooks for the neupogen (filgrastim) global market report 2025 industry through 2025-2034 with The Business Research Company’s reliable data and in-depth research
What is the Projected CAGR for the Neupogen (filgrastim) Market Size from 2025 to 2034?
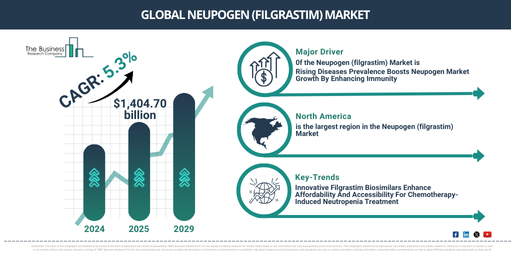
The market for neupogen (filgrastim) has significantly expanded in past years and is projected to continue this trend, with its value expected to increase from $1,330.69 million in 2024 to $1,404.70 billion in 2025, representing a compound annual growth rate (CAGR) of 5.6%. This upward trend in the historical timeline can be linked to a surge in cancer cases, heightened understanding of chemotherapy-induced neutropenia, enhancements in healthcare facilities, early green light for Neupogen biosimilars, and an uptick in worldwide healthcare expenditure.
There is a prediction for a significant increase in the market value of neupogen (filgrastim) in the coming years. By 2029, the market is projected to reach $1,727.05 million, expanding at a compound annual growth rate (CAGR) of 5.3%. This projected increase over the forecast period can be linked to factors such as growing requirement for cost-effective treatments for neutropenia, broader healthcare coverage in fast-growing markets, enhanced governmental backing for biosimilar infiltration, reinforced emphasis on personalized medicine, and an aging demographic. Forecast period trends include escalated investment in research and development for biosimilars, progression in neutropenia treatment drug delivery systems, increased alliances for cancer drug marketing, amplified focus on patient cost relief programs, and the emergence of new-generation G-CSF products.
Download a free sample to assess the report’s scope and structure:
https://www.thebusinessresearchcompany.com/sample.aspx?id=19910&type=smp
Which Factors and External Forces Are Driving Demand in the Neupogen (filgrastim) Market?
The surge of various illnesses is anticipated to fuel the neupogen (filgrastim) market’s expansion. These diseases, caused by infections, inherited traits, environmental elements, or lifestyle decisions, introduce abnormalities in the body’s structure or function, leading to specific symptoms or impacting specific locations. The growing prevalence of these diseases can be attributed to aging populations, inactive lifestyles, increasing pollution, unhealthy diets, and the worldwide dissemination of infectious pathogens. Neupogen (filgrastim) helps manage chemotherapy-induced neutropenia complications by boosting neutrophil formation and strengthening the immune system’s ability to fight off infections in patients with compromised immunity. This makes it key in reducing hospitalization rates, managing infection risks, and maintaining persistent cancer treatment plans for patients receiving intensive therapies. For example, Allergy UK reported in April 2024 that allergies affect more than 21 million UK residents, making it the most frequently reported chronic health issue in 2022. Predictions suggest that half of Europe’s population will have at least one allergy by 2026. Furthermore, Macmillan Cancer Support reported in June 2022 that 3 million people were living with cancer in 2022 in the UK, a figure expected to rise approximately to 3.5 million by 2025 and 4 million by 2030. Hence, the rising occurrence of various diseases propels the growth of the neupogen (filgrastim) market. Government-led healthcare research and development programs are expected to further fuel the expansion of the neupogen (filgrastim) market. These initiatives undertaken by authorities at various levels (local to international) aim to address specific issues, achieve particular objectives, or bring about positive changes in society. Many authorities are spearheading healthcare initiatives that include neupogen (filgrastim) support. As per the Department of Health and Social Care based in the UK in 2022, the UK government disclosed £260 million (US $270.65 million) funding to stimulate healthcare research and manufacturing. This was pledged by BEIS and DHSC to aid NHS-led health research into diagnostics and treatment via new privacy-conserving platforms and clinical research services and £60 million (US $63.60 million) was committed to enhance life sciences manufacturing in the UK. Hence, government-led healthcare research and development initiatives stimulate the neupogen (filgrastim) market.
Which Segments in the Neupogen (filgrastim) Offer the Most Growth?
The neupogen (filgrastim)market covered in this report is segmented –
1) By Drug Type: Biologic; Biosimilar
2) By Indication: Chemotherapy Induced Neutropenia; Chronic Neutropenia; Others
3) By Distribution Channel: Hospital Pharmacies; Retail Pharmacies; Online Pharmacies
Request customized data on this market:
https://www.thebusinessresearchcompany.com/customise?id=19910&type=smp
What Are the Fastest-Growing Geographies in the Neupogen (filgrastim) Market?
North America was the largest region in the neupogen (filgrastim) market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the neupogen (filgrastim) market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
Which Cutting-Edge Market Trends Are Expected to Drive theNeupogen (filgrastim) Market’s Growth?
The principal trend in the Neupogen (filgrastim) market is concentrated on developing innovative filgrastim biosimilar products as an efficient and budget-friendly substitute for the treatment of chemotherapy-induced neutropenia. These biosimilar solutions are biological medications that strongly mirror the original filgrastim (Neupogen) in safety, efficacy, and structure. Their principal purpose is to stimulate neutrophil production in patients receiving chemotherapy to prevent neutropenia. For example, Amneal Pharmaceuticals and Kashiv Biosciences, both US-centric companies, introduced Releuko (filgrastim-ayow), a biosimilar referencing Neupogen (filgrastim), to the US market in November 2022. Available in single-dose vials and prefilled syringes for intravenous and subcutaneous administrations, Releuko provides dosage flexibility to healthcare providers. Its importance lies in providing a budget-friendly therapeutic option for chemotherapy-induced neutropenia, increasing the accessibility of vital oncology care, and meeting the growing demand for biosimilars in the market.
View the full report here:
https://www.thebusinessresearchcompany.com/report/neupogen-filgrastim-global-market-report-
What Are the Key Elements That Define the Neupogen (filgrastim) Market?
Neupogen (filgrastim) is a recombinant granulocyte colony-stimulating factor (G-CSF) that stimulates the production of white blood cells in the bone marrow. It is used to prevent infection in patients with neutropenia caused by chemotherapy, bone marrow transplantation, or certain medical conditions.
Purchase the full report and get a swift delivery:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=19910
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Get in touch with us:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at info@tbrc.info
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model