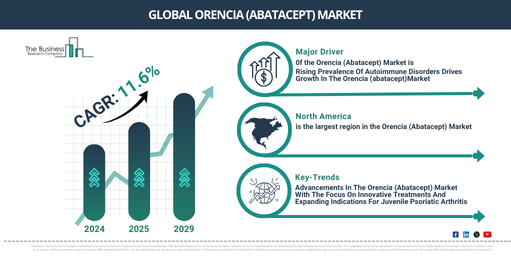
Major Drivers Propelling the Growth oh theOrencia (Abatacept) Market Forward: Rising Prevalence Of Autoimmune Disorders Drives Growth In The Orencia (abatacept)Market
Discover trends, market shifts, and competitive outlooks for the orencia (abatacept) global market report 2025 industry through 2025-2034 with The Business Research Company’s reliable data and in-depth research
What Are the Projected Market Size and Growth Rates for the Orencia (Abatacept) Market From 2025 To 2029?
In recent times, there has been a notable expansion in the market size of Orencia, also known as Abatacept. The market which was valued at $2,727.21 million in 2024, is projected to increase to $3,048.68 million in 2025, indicating a compound annual growth rate (CAGR) of 11.8%. This significant growth during the historic period can be ascribed to several factors including a rising incidence of autoimmune diseases like rheumatoid arthritis and psoriatic arthritis, an escalated demand for biologic treatments, the development of new formulations like subcutaneous abatacept for simpler administration. Other contributing factors are growing awareness and early detection of autoimmune disorders, as well as improved healthcare access.
Anticipations are set for a brisk expansion of the orencia (abatacept) market in the coming years, with it projected to rise to the value of $4,733.52 million by 2029, reflecting a compound annual growth rate (CAGR) of 11.6%. The propelled growth during the projection period can be equated to the escalating acceptance of personalized medicine, growing inclination of patients towards less intrusive subcutaneous administration methods, burgeoning expansion in developing markets due to improved healthcare accessibility, and the continuous transition towards biologic treatments for their amplified efficacy and safety characteristics. Significant trends within the forecast duration encompass the enhancement of reimbursement circumstances in mature markets, a swing towards biosimilars and cost-effective equivalents, collaboration, and product approvals.
Download a free sample to assess the report’s scope and structure:
https://www.thebusinessresearchcompany.com/sample.aspx?id=19915&type=smp
How Are Key Drivers in the Industry Acting as Catalysts for the Growth of the Orencia (Abatacept) Market?
The escalation in autoimmune disorders, where the immune system erroneously targets the body’s own cells, tissues, or organs leading to severe chronic conditions like rheumatoid arthritis or multiple sclerosis, is anticipated to fuel the orencia (abatacept) market’s development. Various factors contribute to this escalation, including genetic predisposition, environmental triggers, and enhanced awareness and diagnostic techniques, resulting in more case identifications. Orencia (abatacept) plays a significant role in managing these diseases by selectively inhibiting T cell stimulation, hence reducing inflammation and the immune system’s attack on normal tissues. In March 2024, the US-based National Health Council reported that autoimmune diseases affected approximately 50 million Americans, escalating swiftly at a rate of 3-12% annually. Also, global multiple sclerosis cases noted a 30% increase in 2022 compared to the last decade. Consequently, the surge in autoimmune disorders is catalyzing the growth of the sports orencia (abatacept) market. Significant advancement in personalized medicine, a customized healthcare approach focusing on patients’ genetic composition, environment, and lifestyle, will spur the Orencia (abatacept) market’s expansion. This progression in personalized medicine comes with the advancement in genomic technologies, enhanced understanding of molecular biology, increased accessibility to precision diagnostic tools, and demand for targeted therapies for better patient outcomes with reduced side effects. Orencia (abatacept) propels the progress of personalized medicine by specifically targeting immune system pathways in autoimmune diseases, facilitating tailored treatments that optimize therapeutic outcomes based on individuals’ unique immune responses and disease traits. According to the Personalized Medicine Coalition, a representative organization in the US, the FDA approved personalized medicines for 34% of new drugs in 2022, and during the last eight years, they approved at least 25% of new drugs. Hence, the surge in personalized medicine is driving the Orencia (abatacept) market.
Which Segments in the Orencia (Abatacept) Offer the Most Growth?
The orencia (abatacept)market covered in this report is segmented –
1) By Type: Prefilled Syringe; Vial
2) By Application: Rheumatoid Arthritis; Juvenile Idiopathic Arthritis; Psoriatic Arthritis
3) By Distribution Channel: Hospital Pharmacies; Retail Pharmacies; Online Pharmacies
Request customized data on this market:
https://www.thebusinessresearchcompany.com/customise?id=19915&type=smp
What Are the Fastest-Growing Geographies in the Orencia (Abatacept) Market?
North America was the largest region in the orencia (abatacept) market in 2024. The regions covered in the orencia (abatacept) market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
What Are the Current Market Growth and Trends in the Orencia (Abatacept) Industry?
A predominant trend in the orencia (abatacept) industry is the increasing encapsulation towards creating ground-breaking medications to advance treatment efficacy and patient outcomes, and bridge the gap in dealing with autoimmune conditions. Abatacept is a bio-pharmaceutical used to treat juvenile psoriatic arthritis (JPsA), an uncommon autoimmune disorder prevalent in children and teenagers that triggers joint inflammation and skin ailments. Bristol Myers Squibb, an American pharmaceutical firm, disclosed the sanction of Biologics Licensing Application (sBLA) for abatacept (Orencia) intended for the therapy of juvenile psoriatic arthritis (JPsA) in patients belonging to the 2 to 17 years age group by the Food and Drug Administration, a US government entity in October 2023. The approval relied on information obtained from regulated tests on both adult and juvenile populations. The studies particularly utilized pharmacokinetic information obtained from adult rheumatoid arthritis and psoriatic arthritis patients, supplemented by safety information from clinical trials administered on a group of children aged 2 to 17 years suffering from polyarticular JIA.
View the full report here:
https://www.thebusinessresearchcompany.com/report/orencia-abatacept-global-market-report
What Are the Key Elements That Define the Orencia (Abatacept) Market?
Orencia (abatacept) is a prescription medication used to treat certain autoimmune diseases. It is classified as a selective T-cell co-stimulation modulator, which suppresses overactive immune responses that cause inflammation and tissue damage.
Purchase the full report and get a swift delivery:
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=19915
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Get in touch with us:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at info@tbrc.info
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model