Market Trends Influencing Strategic Decisions in the Paroxysmal Nocturnal Hemoglobinuria (PNH) And Atypical Hemolytic Uremic Syndrome (aHUS) Industry: Development Of Complement Inhibitors Revolutionizing PNH And aHUS Treatment With Enhanced Patient Convenience And Outcomes
We’ve updated all our reports with current data on tariff changes, trade developments, and supply chain shifts affecting key industries.
What Is the Current and Projected Market Size of the Paroxysmal Nocturnal Hemoglobinuria (PNH) And Atypical Hemolytic Uremic Syndrome (aHUS) Market Through 2034?
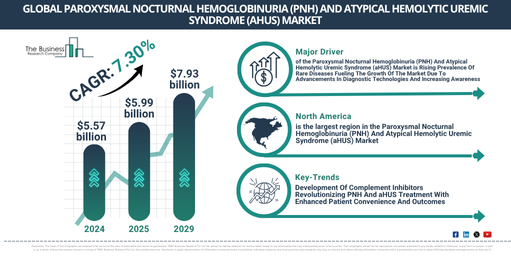
Recent years have seen a robust growth in the market size of Paroxysmal Nocturnal Hemoglobinuria (PNH) and Atypical Hemolytic Uremic Syndrome (aHUS). The market, currently standing at $5.57 billion in 2024, is projected to expand to $5.99 billion in 2025 with a 7.4% compound annual growth rate (CAGR). The growth in the previous years has been linked to the rising occurrence of PNH and aHUS, increased funding in the research of rare diseases, a growing demand for targeted therapies, heightened awareness about PNH and aHUS, and an expanding adoption of complement inhibitors.
The market size for paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS) is projected to experience robust growth over the next years, reaching $7.93 billion by 2029, with a Compound Annual Growth Rate (CAGR) of 7.3%. This expansion during the forecast period is credited to growing regulatory endorsement of new treatments, increased collaborations between biotech corporations and research institutes, and escalating demand for personalized medicine. Furthermore, there is an upward trend in investing in startups focusing on rare diseases, and a surge in the number of clinical trials and research studies. Key trends for the projection period encompass progress in complement inhibition treatments, the incorporation of gene therapy methods, the evolution of personalized medicine, the emergence of technology-led diagnostic instruments, and the merging of technology for monitoring patients remotely.
Download a free sample to assess the report’s scope and structure:
https://www.thebusinessresearchcompany.com/sample.aspx?id=24357&type=smp
What Are the Major Market Drivers Behind the Rising Adoption of Paroxysmal Nocturnal Hemoglobinuria (PNH) And Atypical Hemolytic Uremic Syndrome (aHUS) Market?
The growth of the market for paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS) is anticipated to be fuelled by the increasing occurrence of rare diseases. These are medical conditions, typically affecting fewer than 200,000 individuals, and are often distinguished by their complexity, severity, and the requirement for specialised treatment. The rise in rare diseases can be linked to advancements in diagnostic technologies that facilitate better detection and identification of earlier underdiagnosed or unreported conditions. Notably, PNH and aHUS contribute to our understanding and management of rare diseases, providing insights into the pathophysiology and potential therapeutic targets for conditions with few treatment options. For example, in November 2024, the Food and Drug Administration, a US-based federal agency, reported that more than 7,000 rare diseases affected over 30 million individuals in the US. Thus, with the growing prevalence of these rare diseases, the growth of the PNH and aHUS market is being driven forward.
Which Key Market Segments Comprise the Paroxysmal Nocturnal Hemoglobinuria (PNH) And Atypical Hemolytic Uremic Syndrome (aHUS) Market and Drive Its Revenue Growth?
The paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS) market covered in this report is segmented –
1) By Type: Soliris, Ultomiris
2) By Disease Type: Paroxysmal Nocturnal Hemoglobinuria(PNH), Atypical Hemolytic Uremic Syndrome(aHUS)
3) By Treatment Type: Complement Inhibitors, Plasma Exchange Or Infusion, Other Symptomatic Treatments
4) By Diagnosis Method: Clinical Diagnosis, Genetic Testing, Biomarker Testing
5) By End-User: Hospitals, Specialty Clinics, Research Institutes, Other Users
Subsegments:
1) By Soliris: Eculizumab For PNH, Eculizumab For aHUS, Eculizumab Biosimilars
2) By Ultomiris: Ravulizumab For PNH, Ravulizumab For aHUS, Ravulizumab Extended-Interval Dosing
Request customized data on this market:
https://www.thebusinessresearchcompany.com/sample.aspx?id=24357&type=smp
Which Areas Are Leading Regions in the Paroxysmal Nocturnal Hemoglobinuria (PNH) And Atypical Hemolytic Uremic Syndrome (aHUS) Market Expansion Across the Globe?
North America was the largest region in the paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS) market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS) market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
What Are the Strategic Trends Steering the Paroxysmal Nocturnal Hemoglobinuria (PNH) And Atypical Hemolytic Uremic Syndrome (aHUS) Market Direction?
Leading companies in the paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS) market are concentrating on the creation of revolutionary products like complement inhibitors, to enhance patient care and treatment results. Complement inhibitors act as therapeutic tools that interrupt specific parts of the immune system’s complement system, aiding in the removal of pathogens and damaged cells. For instance, in December 2023, the Switzerland-based pharmaceutical giant, Novartis AG, secured U.S. Food and Drug Administration (FDA) approval for their product Fabhalta (iptacopan). This is the first dogged oral treatment for adults with paroxysmal nocturnal hemoglobinuria, offering superior hemoglobin enhancement without the necessity for transfusions. This groundbreaking complement inhibitor operates by restricting Factor B in the alternative complement pathway, thereby offering extensive control of both intravascular and extravascular hemolysis. Its oral intake simplifies disease control and enhances patient life quality by removing the difficulties correlated with regular infusions.
View the full report here:
How Is the Paroxysmal Nocturnal Hemoglobinuria (PNH) And Atypical Hemolytic Uremic Syndrome (aHUS) Market Conceptually Defined?
Paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS) refer to rare, life-threatening blood disorders caused by uncontrolled activation of the complement system, a part of the immune system. Paroxysmal nocturnal hemoglobinuria (PNH) leads to the destruction of red blood cells, while atypical hemolytic uremic syndrome (aHUS) causes blood clots in small blood vessels, primarily affecting the kidneys and other vital organs.
Purchase the full report and get a swift delivery:
https://www.thebusinessresearchcompany.com/customise?id=24357&type=smp
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Get in touch with us:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at info@tbrc.info
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model