Point-Of-Care Molecular Diagnostics Growth Forecast 2025-2034: Trends, Opportunities, and Key Insights You Need to Know
Updated 2025 Market Reports Released: Trends, Forecasts to 2034 – Early Purchase Your Competitive Edge Today!
What is the Predicted CAGR for the Point-Of-Care Molecular Diagnostics Market Over the Forecast Period of 2025 to 2034?
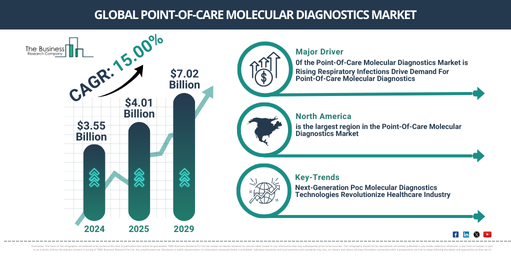
In recent times, there has been a swift expansion in the market size of point-of-care molecular diagnostics. The projections show an increase from $3.55 billion in 2024 to $4.01 billion in 2025, implying a compound annual growth rate (CAGR) of 12.9%. This upsurge during the past era occurs because of the growing requirement for immediate disease identification, the escalation of infectious disease occurrences, the widening of telemedicine and distant testing, regulatory backing for point-of-care diagnostics, and the necessity for individualized and precision medicine.
The market size of point-of-care molecular diagnostics is projected to undergo swift expansion in the coming years, reaching a valuation of $7.02 billion in 2029, with a compound annual growth rate (CAGR) of 15.0%. This growth during the forecasted period can be linked to factors such as the shrinkage of diagnostic devices, augmentation of point-of-care testing for cancer and genetic ailments, specialty molecular diagnostics for a plethora of applications, incorporation of artificial intelligence and machine learning for data interpretation, and the surveillance of emerging infectious diseases. Key trends expected during the forecast period comprise lab-on-a-chip and microfluidic technologies, digital health and smartphone-centric diagnostics, readiness for point-of-care COVID-19 and other pandemics, predictive diagnostics driven by AI, and molecular testing based on CRISPR.
What Key Drivers Are Accelerating the Growth of the point-of-care molecular diagnostics Market During the Forecast Period?
The escalating prevalence of infectious and respiratory diseases is anticipated to majorly fuel the expansion of the point-of-care molecular diagnostics market. Respiratory infections, triggered by microorganisms such as viruses or bacteria, impact the respiratory system. These pathogens can be disseminated via coughing, sneezing, or direct contact. The assortment of treatments for severe respiratory diseases comprises various inhalers, oral medications, intravenous therapies, and point-of-care molecular diagnostics to treat the patient. For example, data from the Office for National Statistics, a respected national statistics organization in the UK, revealed that in England, the infection rate rose for individuals aged 35 to 69 years and also escalated for those in school years 12 to 24 years, by the end of the week concluding December 9, 2022. As a result, the swift surge in infectious and respiratory diseases and associated fatalities will stimulate the point-of-care molecular diagnostics market.
Request Your Free Point-Of-Care Molecular Diagnostics Market Report Sample Now!
https://www.thebusinessresearchcompany.com/sample.aspx?id=7046&type=smp
Who Are the Top Companies Driving Innovation and Growth in the Point-Of-Care Molecular Diagnostics Market?
Major companies operating in the point-of-care molecular diagnostics market include:
• Abbott Laboratories_x000D_
• F Hoffmann-La Roche Ltd._x000D_
• BioMérieux SA_x000D_
• Danaher Corporation_x000D_
• Quidel Corporation_x000D_
What Key Trends Are Currently Impacting the Point-Of-Care Molecular Diagnostics Market’s Development?
Technological advancement is becoming a significant trend in the point-of-care molecular diagnostics market. The leading companies in this market are focusing on delivering high-tech solutions to solidify their standing in the market. They are utilizing advanced point-of-care molecular diagnostics technology solutions and related services such as enzyme-linked immunosorbent assay (ELISA), polymerase chain reaction (PCR), mass spectrometry (MS), situ hybridizations, spectral karyotyping imaging, DNA microarrays, and others. These features aim to speed up analysis times and reduce costs when implemented on POC devices. As an example, in May 2023, US firm Sensible Diagnostics introduced a point-of-care PCR system that delivers results in 10 minutes. The company is leveraging the hands-on experience Curative accumulated during the pandemic in the diagnostics industry. The company reported having processed more than two million molecular point-of-care COVID-19 tests, and its PCR platform can produce results in as little as 10 minutes.
Pre-order Your Report for Quick and Easy Delivery!
Which Key Segments Stand Out in Understanding the Composition of the Point-Of-Care Molecular Diagnostics Market?
The point-of-care molecular diagnostics market covered in this report is segmented –
1) By Product And Service: Assays And Kits, Instruments And Analyzers, Software And Services
2) By Technology: Reverse Transcription – Polymerase Chain Reaction (RT-PCR), In Situ Hybridization, Sequencing
3) By Application: Respiratory Diseases, Hospital Acquired Infections (HAIs), Cancer/Oncology, Hepatitis, Hematology
4) By End-User: Decentralized Labs, Hospitals, Home Care, Assisted Living Healthcare Facilities
Subsegments:
1) By Assays And Kits: Nucleic Acid Amplification Tests (NAATs), Rapid Diagnostic Tests, Molecular Detection Kits, Reagents And Consumables
2) By Instruments And Analyzers: PCR Machines, Microfluidic Devices, Sequencers, Point-of-Care Analyzers
3) By Software And Services: Data Management Software, Cloud-Based Solutions, Technical Support And Maintenance Services, Consultation And Training Services
Which Geographical Regions Are Pioneering Growth in the Point-Of-Care Molecular Diagnostics Market?
North America was the largest region in the point-of-care molecular diagnostics market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the point-of-care molecular diagnostics market report include Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa.
What Are the Defining Aspects of the Point-Of-Care Molecular Diagnostics Market Landscape?
Point-of-care (POC) molecular diagnostics refers to tests that detect the presence of specific nucleic acids in a clinical specimen, such as feces, saliva, urine, blood, and tissue. This molecular diagnostic is based on seeing targeted portions of microbial genetic material, DNA or RNA. It is utilized by the healthcare sector to detect emergency usage authorization to diagnose diseases. Point-of-care (POC) molecular diagnostic is used to detection of disease antigens or antibodies in human samples, such as mononucleosis, influenza, and group A streptococcus (GAS).
Browse Through More Similar Reports By The Business Research Company:
Point of Care Ultrasound Global Market Report 2024
https://www.thebusinessresearchcompany.com/report/point-of-care-ultrasound-global-market-report
Veterinary Point Of Care Diagnostics Global Market Report 2024
Point of Care Diagnostics Global Market Report 2024
https://www.thebusinessresearchcompany.com/report/point-of-care-diagnostics-global-market-report
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Contact us at:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at info@tbrc.info
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
Found this article helpful? Share it on:
[KClientError] [REQ_ERR: OPERATION_TIMEDOUT] [KTrafficClient] Something is wrong.