Global Point Of Care Molecular Diagnostics Market Size, Share, Trends And Drivers 2023-2032
The Business Research Company’s global market reports are now updated with the latest market sizing information for the year 2023 and forecasted to 2032
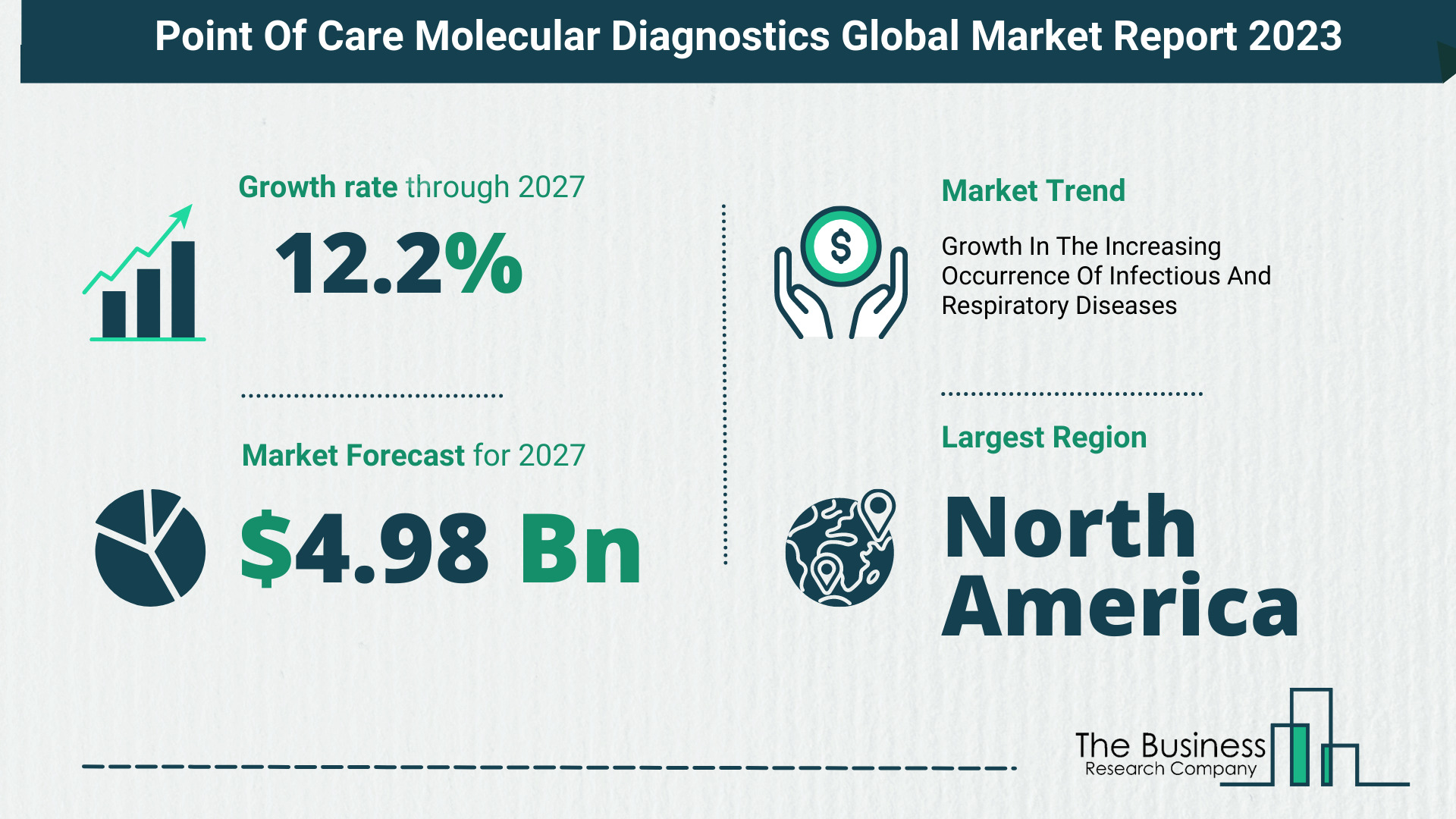
North America held the largest point-of-care molecular diagnostics market share in 2022.
The global point-of-care molecular diagnostics market size will grow from $2.8 billion in 2022 to $3.14 billion in 2023 at a compound annual growth rate (CAGR) of 12.1%. The point-of-care molecular diagnostics market size is expected to grow to $4.98 billion in 2027 at a CAGR of 12.2%.
Major Driver In The Point Of Care Molecular Diagnostics Market – Growth In The Increasing Occurrence Of Infectious And Respiratory Diseases
For example, an annual Report on MCCD (Medical Certification of Cause of mortality) 2020 published in May 2020 shows that respiratory system disorders are the second leading cause of mortality, accounting for 10% of all medically certified causes of death (88.7%). Furthermore, the number of deaths caused by respiratory illnesses increased from 152,311 to 181,160 between 2019 and 2020. As a result, the rapid occurrence of infectious and respiratory disorders, as well as death, will drive the market for point-of-care-molecular diagnostics.
View More On The Point Of Care Molecular Diagnostics Market Report 2023 – https://www.thebusinessresearchcompany.com/report/point-of-care-molecular-diagnostics-global-market-report
Key Point Of Care Molecular Diagnostics Market Segments
1) By Product And Service: Assays And Kits, Instruments And Analyzers, Software And Services
2) By Technology: Reverse Transcription – Polymerase Chain Reaction (RT-PCR), In Situ Hybridization, Sequencing
3) By Application: Respiratory Diseases, Hospital Acquired Infections (HAIs), Cancer/Oncology, Hepatitis, Hematology
4) By End-User: Decentralized Labs, Hospitals, Home Care, Assisted Living Healthcare Facilities
Recent Point Of Care Molecular Diagnostics Market Trend – Technological Advancement
For example, Abbott Laboratories, a medical device firm based in the United States, introduced a molecular point-of-care test for detecting novel COVID-19 (COVID-19) in March 2020. According to the manufacturer, the device produces positive results in five minutes and negative results in thirteen minutes. This test will be performed using the ID NOWTM platform, which uses isothermal nucleic acid amplification technology to get molecular results in minutes.
Point Of Care Molecular Diagnostics Market Prominent Players
Major players in the point-of-care molecular diagnostics market are Abbott Laboratories, F Hoffmann-La Roche Ltd., BioMérieux SA, Danaher Corporation, Quidel Corporation, Qiagen Gmbh, Biocartis NV, Nova Biomedical, Thermo Fisher Scientific, Dickinson and Company, Mesa Biotech Inc., OraSure Technologies Inc., Bio-Rad Laboratories Inc., Sysmex, Siemens Healthineers, Bayer HealthCare Pharmaceuticals LLC, Alere Inc., Lucira Health Inc., Cue Health, OpGen Inc., Binx Health Inc., Molbio Diagnostics Pct Ltd., Genomadix, Visby Medical, QuikPath PTE Ltd., QuantuMDx Group Ltd and Sekisui Medical Co Ltd.
Request A Sample Of The Global Point Of Care Molecular Diagnostics Market Report 2023:
https://www.thebusinessresearchcompany.com/sample.aspx?id=7046&type=smp
The Point Of Care Molecular Diagnostics Global Market Report 2023 provides a comprehensive overview on the point of care molecular diagnostics market size, trends and drivers, opportunities, strategies, and companies analysis. The countries covered in the point of care molecular diagnostics market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, the UK, and the USA, and the major seven regions are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, the Middle East, and Africa.
Point-of-care (POC) molecular diagnostics is the detection of specific nucleic acids in clinical specimens such as feces, saliva, urine, blood, and tissue. This molecular diagnosis is based on observing specific segments of microbial genetic material, such as DNA or RNA. It is used in the healthcare industry to detect emergency usage authorisation in order to diagnose disorders. Point-of-care (POC) molecular diagnostics are employed in human samples to detect disease antigens or antibodies, such as mononucleosis, influenza, and group A streptococcus (GAS).
View More Related Reports –
Poc Hba1C Testing Global Market Report 2023
https://www.thebusinessresearchcompany.com/report/poc-hba1c-testing-global-market-report
POC Medical Imaging Global Market Report 2023
https://www.thebusinessresearchcompany.com/report/poc-medical-imaging-global-market-report
Point-Of-Care Diagnostics Devices And Equipment Global Market Report 2023
https://www.thebusinessresearchcompany.com/report/point-of-care-diagnostics-devices-and-equipment-global-market-report
Contact us at:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at [email protected]
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model