What Is The Forecast Growth Rate For The Real World Evidence Solutions Market?
The Business Research Company’s market reports offer an in-depth analysis on the market’s growth potential, major drivers, key trends and more.
The real-world evidence solutions market has witnessed substantial growth in recent years, with a promising trajectory for the future.
Market Expansion
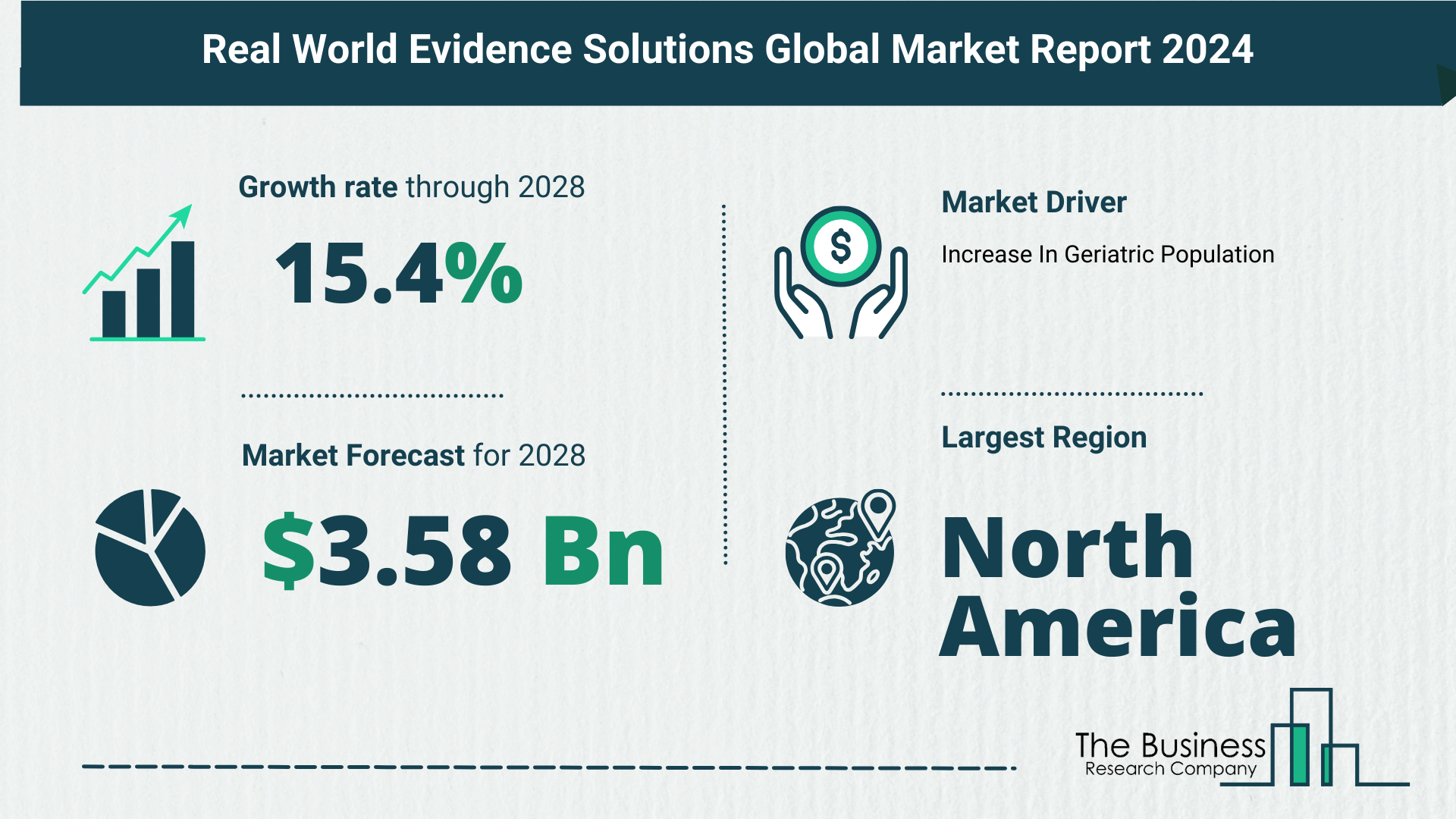
- Growing from $1.75 billion in 2023 to $2.02 billion in 2024, showcasing a robust CAGR of 15.3%.
- Projections for the future anticipate reaching $3.58 billion in 2028, sustaining a strong CAGR of 15.4%.
Historical Drivers
- Growth in pharmaceutical and healthcare sectors.
- Demand for post-market surveillance and drug safety.
- Expansion of electronic health records and data.
- Adoption in clinical research and outcomes assessment.
- Regulatory standards for real-world evidence (RWE).
Catalysts for Future Growth
Customized RWE Solutions
- The forecast period anticipates tailored RWE solutions for specific therapeutic areas.
- Meeting the unique needs of different medical domains is expected to drive market growth.
Expansion of Real-World Data Networks
- The growth trajectory includes an expansion of real-world data networks.
- A broader network enhances the depth and breadth of data available for evidence generation.
Innovation in Evidence Generation
- The forecast sees an emphasis on innovation in real-world evidence generation.
- Novel approaches aim to enhance the quality and relevance of evidence in healthcare decision-making.
Collaboration in Drug Development
- The adoption of RWE in drug development and access is a significant driver.
- Collaborative efforts between healthcare and technology sectors are expected to shape the future landscape.
Emerging Trends in the Forecast Period
AI-Driven Predictive Analytics
- AI-driven predictive analytics in RWE is a notable trend.
- Advanced technologies contribute to more accurate predictions and insights.
Regulatory Compliance
- Ensuring regulatory compliance in RWE data privacy and security is gaining prominence.
- Stricter adherence to regulations enhances trust and reliability in real-world evidence.
Personalized RWE Solutions
- Personalized RWE solutions for healthcare providers are becoming prevalent.
- Tailoring evidence to specific healthcare contexts improves its applicability.
Transparency in Data Sharing
- Transparency in data sharing and RWE findings is a growing trend.
- Open communication fosters trust and collaboration in the healthcare ecosystem.
Integration in Drug Pricing
- The integration of RWE in drug pricing and reimbursement is gaining traction.
- Evidence-based pricing strategies contribute to a more sustainable healthcare system.
Government Support as a Driving Force
Fostering a Favorable Environment
- Government support plays a pivotal role in driving market growth.
- Efforts to raise public awareness create a conducive environment for the adoption of RWE solutions.
Global Initiatives
- International initiatives, such as the UK government’s investment in medical device approval bodies, showcase global support.
- National agencies, like Romania’s National Agency for Health Infrastructure Development, contribute to a cohesive healthcare ecosystem.
Noteworthy Market Players
Maxis Clinical Sciences’ RWE Solutions
- Maxis Clinical Sciences introduces a new RWE Solutions service.
- Optimizing evidence-based medicine, the service combines various real-world data sources.
Cerner Corporation’s Strategic Acquisition
- Cerner Corporation enters the RWE solutions market with the acquisition of Kantar Health.
- The $375 million deal brings together Cerner’s real-world data and Kantar Health’s life sciences expertise.
Market Segmentation
- Component
- Services
- Data Sets
- Clinical Setting Data
- Claims Data
- Pharmacy Data
- Patient Powered Data
- Therapeutic Area
- Oncology
- Cardiovascular
- Neurology
- Immunology
- Other Therapeutic Areas
- Application
- Drug Development And Approvals
- Medical Device Development And Approvals
- Reimbursement/Coverage And Regulatory Decision Making
- Post Market Safety And Adverse Events Monitoring
- End-Users
- Pharmaceutical And Medical Devices Companies
- Healthcare Providers
- Healthcare Payers
- Other End-Users
Regional Dynamics
North America’s Leading Role
- North America dominated the real-world evidence solutions market in 2023.
- Robust healthcare infrastructure and research capabilities contribute to the region’s prominence.
Asia-Pacific’s Growth Potential
- Asia-Pacific emerges as the fastest-growing region in the forecast period.
- Increasing disease burden and emerging markets contribute to the region’s anticipated growth.
In conclusion, the real-world evidence solutions market is poised for continued growth, fueled by government support, innovative solutions, and strategic collaborations. Emerging trends and evolving technologies promise to reshape the landscape, providing a solid foundation for evidence-based decision-making in the healthcare industry.
Read More On The Real World Evidence Solutions Market Report 2024 – https://www.thebusinessresearchcompany.com/report/real-world-evidence-solutions-global-market-report
Request for A Sample Of The Global Real World Evidence Solutions Market Report:
https://www.thebusinessresearchcompany.com/sample_request?id=6930&type=smp