Rotavirus Prophylaxis Market Growth Forecast: Exploring Trends and Opportunities for the Next Decade
Updated 2025 Market Reports Released: Trends, Forecasts to 2034 – Early Purchase Your Competitive Edge Today!
What is the Predicted CAGR for the Rotavirus Prophylaxis Market Over the Forecast Period of 2025 to 2034?
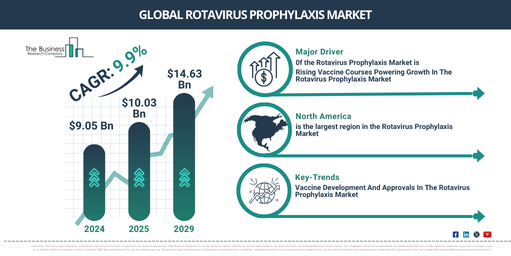
The size of the market for rotavirus prophylaxis has seen swift expansion in the past few years. There will be a leap from $9.05 billion in 2024 to $10.03 billion in 2025, presenting a compound annual growth rate (CAGR) of 10.8%. The remarkable growth during the historic period is the result of accessibility and fairness of vaccines, emergence of new rotavirus strains, investments in research and development, and readiness in public health.
The market size for rotavirus prophylaxis is predicted to experience significant expansion in the coming years, reaching a valuation of $14.63 billion in 2029 with a 9.9% compound annual growth rate (CAGR). Factors contributing to this projected growth during the forecast period include the burden of gastrointestinal diseases, worldwide expansion, immunization initiatives, and confidence in vaccines. The forecast period is also expected to witness the emergence of main trends, including next-generation vaccines, combination vaccines, innovative methods of vaccine delivery, and global collaborations for immunization.
What Factors Are Propelling the Growth of the Rotavirus Prophylaxis Market from 2025 to 2034?
The surge in vaccine doses is poised to fuel the expansion of the rotavirus prophylaxis market. Essentially, a vaccine dose is a particular quantity of vaccine planned to be administered to an individual to yield immunity against a specific disease. The dosage triggers an immunological reaction, offering exceptional safeguards against a rotavirus infection. This boosted immunity reduces disease vulnerability and enhances the vaccine’s overall effectiveness. For example, as reported by the United Nations International Children’s Emergency Fund (UNICEF) in January 2022, approximately 5.71 million courses of the RV1-5 frozen rotavirus vaccine were manufactured in 2022, with an estimated increase of up to 9.31 million courses by 2028. Similarly, 5.29 million courses of RV5-2 lyophilized rotavirus vaccines were manufactured in 2022, with an estimated surge of up to 6.13 million courses by 2028. As a result, the growing volume of vaccines will spearhead the rotavirus prophylaxis market. In terms of market impetus for the Rotavirus Prophylaxis Market, integral funding contributes to the advancement of the rotavirus prophylaxis market. Institutional funding pertains to the allocation of monetary, effort, or time resources to meet a need, fund a program, or conduct a project. The rise in rotavirus cases has drawn funding from both government and private entities. This funding provides both financial and technical aid throughout different developmental stages of rotavirus vaccines and oral medication. Indiana University, a public research university based in the US, for example, received a $1.2 million grant from venture capital firm GIVAX Inc. in April 2022 to develop a novel technology for a combined oral rotavirus-norovirus vaccine for infants. This technology extends the widely available rotavirus vaccine to add safeguards against norovirus, a highly contagious virus causing severe vomiting and diarrhea in young children. Consequently, the broadening of funding availability will bolster the rotavirus prophylaxis market.
Get Your Free Sample of the Global Rotavirus Prophylaxis Market Report Now!
https://www.thebusinessresearchcompany.com/sample.aspx?id=13045&type=smp
Who Are the Top Companies Driving Innovation and Growth in the Rotavirus Prophylaxis Market?
Major companies operating in the rotavirus prophylaxis market are:
• Pfizer Inc._x000D_
• Johnson & Johnson_x000D_
• Merck & Co._x000D_
• Novartis AG_x000D_
• Sanofi SA_x000D_
What Key Trends Are Currently Impacting the Rotavirus Prophylaxis Market’s Development?
Leading firms in the rotavirus prophylaxis market are focused on creating vaccines that fulfill client needs. Approval for these vaccines necessitates a thorough procedure that involves numerous stages of development and testing. These authorizations are crucial for confirming the safety and effectiveness of vaccines in preventing this viral infection, especially in babies and young children. For instance, in November 2022, the UK-based pharmaceutical and biotechnology corporation GlaxoSmithKline PLC declared that the Food and Drug Administration (FDA) had authorized a liquid formulation of ROTARIX (Rotavirus Vaccine, Live and Oral) that obstructs rotavirus gastroenteritis instigated by G1 and non-G1 types (G3, G4 and G9) in infants. This innovative liquid-only presentation of the oral-dosing applicator does not need to be reconstituted prior to administration. Both the lyophilized and liquid versions of ROTARIX are given orally in two doses and consist of the same live, human-tamed rotavirus strain produced using similar methods.
Get Instant Access to the Global Rotavirus Prophylaxis Market Report with Swift Delivery!
https://www.thebusinessresearchcompany.com/report/rotavirus-prophylaxis-global-market-report
Which Primary Segments of the Rotavirus Prophylaxis Market Are Driving Growth and Industry Transformations?
The rotavirus prophylaxis market covered in this report is segmented –
1) By Treatment: Rotarix, Rotavac, Rotavin-M1, Oral Rehydration Fluid, Other Treatments
2) By Route of Administration: Oral, Parenteral, Other Route of Administrations
3) By Distribution Channel: Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Other Distribution Channels
4) By End-Users: Hospitals, Specialty Clinics, Homecare, Other End-Users
Subsegments:
1) By Rotarix: Single Dose, Multi-Dose
2) By Rotavac: Liquid Formulation, Freeze-Dried Formulation
3) By Rotavin-M1: Liquid Formulation, Freeze-Dried Formulation
4) By Oral Rehydration Fluid: Electrolyte Solutions, Glucose-Based Solutions
5) By Other Treatments: Supportive Care, Antidiarrheal Medications
Which Geographical Regions Are Pioneering Growth in the Rotavirus Prophylaxis Market?
North America was the largest region in the rotavirus prophylaxis market in 2024. The regions covered in rotavirus prophylaxis market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa.
What Are the Key Characteristics That Define the Rotavirus Prophylaxis Market?
Rotavirus prophylaxis refers to the prevention of rotavirus infection through vaccination, which protects infants and young children from a highly infectious illness that causes stomach and bowel inflammation. The purpose is to protect from person from any severity.
Browse Through More Similar Reports By The Business Research Company:
Meningococcal Vaccines Global Market Report 2024
https://www.thebusinessresearchcompany.com/report/meningococcal-vaccines-global-market-report
Cancer Vaccines Global Market Report 2024
https://www.thebusinessresearchcompany.com/report/cancer-vaccine-global-market-report
Companion Animal Veterinary Vaccines Global Market Report 2024
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Contact us at:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at [email protected]
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
Found this article helpful? Share it on: