Sterile Endotracheal Tube Market Insights 2025-2034: Growth Dynamics, Trends, and Strategic Opportunities
Updated 2025 Market Reports Released: Trends, Forecasts to 2034 – Early Purchase Your Competitive Edge Today!
What are the Key Projections for the CAGR of the Sterile Endotracheal Tube Market From 2025 to 2034?
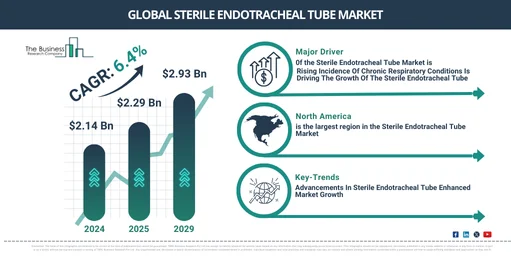
The market size for sterile endotracheal tubes has seen significant growth in recent years. The market is expected to increase from $2.14 billion in 2024 to $2.29 billion in 2025, exhibiting a compound annual growth rate (CAGR) of 6.7%. Factors contributing to this growth during the historical period include heightened demand for trustworthy intubation, increased knowledge of airway management, a surge in infection control awareness, enhanced patient safety norms, and a shift towards patient-oriented care methods.
Over the upcoming years, the sterile endotracheal tube market is projected to experience robust growth. It’s anticipated to rise to $2.93 billion in 2029, delivering a compound annual growth rate (CAGR) of 6.4%. This growth in the projected period can be credited to factors such as the development of healthcare infrastructure, rise in emergency medical services (EMS), escalation in chronic obstructive pulmonary disease cases, advent of disposable endotracheal tubes, and legislative transformations. Major upcoming trends include the enhancement of materials and coatings, improved visualisation, customisations and personalisations, added safety measures, as well as superior ergonomics and user experiences.
What Combination of Drivers Is Leading to Accelerated Growth in the Sterile Endotracheal Tube Market?
The escalating prevalence of chronic respiratory disorders is predicted to fuel the expansion of the sterile endotracheal tube market. Chronic respiratory disorders are enduring illnesses that impact the lungs and other components of the respiratory system, resulting in continual breathing problems. The escalating prevalence of these disorders can be attributed to elements such as escalating environmental pollution, increased smoking rates, an aging demographic, inactive lifestyles, climate variations, occupational hazards, and socio-economic inequalities. To ensure a sterile airway for mechanical ventilation and to reduce the risk of infection, sterile endotracheal tubes are utilized in chronic respiratory disorders. For instance, a report published by the Australian Institute of Health and Welfare, a government organization based in Australia, showed that in June 2024, there were 56,600 emergency department visits for asthma, equivalent to 230 visits per 100,000 individuals. In 2022, respiratory disorders were either a primary or related cause of 54,776 deaths, accounting for 211 deaths per 100,000 population, or 29% of all deaths in 2020–21. As a result, the escalating prevalence of chronic respiratory disorders is catalyzing the expansion of the sterile endotracheal tube market.
Explore Comprehensive Insights Into The Global Sterile Endotracheal Tube Market With A Free Sample Report:
https://www.thebusinessresearchcompany.com/sample.aspx?id=19721&type=smp
What Are the Key Industry Players Leading the Charge in the Sterile Endotracheal Tube Market’s Growth?
Major companies operating in the sterile endotracheal tube market are Medtronic plc, Cook Medical Incorporated, Teleflex Incorporated, Zoll Medical Corporation, Hollister Incorporated, Avanos Medical Inc., Ambu A/S, Intersurgical Inc., Vyaire Medical Inc., Angiplast Pvt Ltd., Amsino International Inc., Inovet, GPC Medical Ltd., C. R. Bard Inc., Steroplast Healthcare Limited, Hitec Medical Co Ltd., Sterimed Group, Neurovision Medical Products Inc., Novo Klinik-Service GmbH, Well Lead Medical Co Ltd.
How Are Market Trends and Shifts Impacting the Growth Trajectory of the Sterile Endotracheal Tube Market?
Leading companies in the sterile endotracheal tube market are concentrating on the creation of innovative products such as endotracheal tube holders, with an aim to improve patient treatment and make intubation procedures more effective. The endotracheal tube holder is a medical apparatus that holds and steadies an endotracheal (ET) tube post its insertion into a patient’s trachea. To illustrate, Dale Medical Products Inc., a specialty medical device company based in the US, enlarged its range of user-friendly medical device securement solutions by launching the BreezeLock Endotracheal Tube Holder in June 2022. This recent addition provides a soft, flexible neckband devoid of rigid plastic elements, similar to the currently available Stabilock model. The BreezeLock stands out for its Tube Track, facilitating the easy readjustment of the endotracheal tube and guaranteeing uninterrupted access to oral care.
Secure Your Global Sterile Endotracheal Tube Market Report Now for Fast and Efficient Delivery!
https://www.thebusinessresearchcompany.com/report/sterile-endotracheal-tube-global-market-report
How Are the Key Segments of the Sterile Endotracheal Tube Market Driving Opportunities and Innovations?
The sterile endotracheal tubemarket covered in this report is segmented –
1) By Type: No Capsules, Olive Sac, Columnar Sac
2) By Application: Emergency Treatment, Therapy, Other Applications
3) By End Users: Clinics, Hospitals, Ambulatory Surgical Centers, Other End Users
Subsegments:
1) By No Capsules: Standard Endotracheal Tubes, Cuffed Endotracheal Tubes, Uncuffed Endotracheal Tubes
2) By Olive Sac: Olive Sac Endotracheal Tubes With Cuffs, Olive Sac Endotracheal Tubes Without Cuffs, Pediatric Olive Sac Endotracheal Tubes
3) By Columnar Sac: Columnar Sac Endotracheal Tubes With Cuffs, Columnar Sac Endotracheal Tubes Without Cuffs, High-Volume, Low-Pressure Columnar Sac Tubes
What Regions Are Influencing the Dynamics of the Sterile Endotracheal Tube Market?
North America was the largest region in the sterile endotracheal tube market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the sterile endotracheal tube market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
How Is the Definition of the Sterile Endotracheal Tube Market Impacting Future Growth?
A sterile endotracheal tube refers to a medical device used to secure an airway for a patient who is unable to breathe on their own or requires mechanical ventilation. A sterile endotracheal tube is utilized by inserting it into a patient’s trachea to maintain an open airway and facilitate mechanical ventilation while preventing infection through its sterile packaging and handling.
Browse Through More Similar Reports By The Business Research Company:
Integrated Patient Care Systems Global Market Report 2024
Cardiovascular Clinical Trials Global Market Report 2024
DNA Diagnostics Global Market Report 2024
https://www.thebusinessresearchcompany.com/report/dna-diagnostics-global-market-report
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Contact us at:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at info@tbrc.info
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
Found this article helpful? Share it on: