Top 5 Insights From The Mucolipidosis II Market Report 2024
The Business Research Company’s global market reports are now updated with the latest market sizing information for the year 2024 and forecasted to 2033
According to The Business Research Company’s Mucolipidosis II Global Market Report 2023, the mucolipidosis II market is expected to show promising growth in the forecast period.
Market Overview
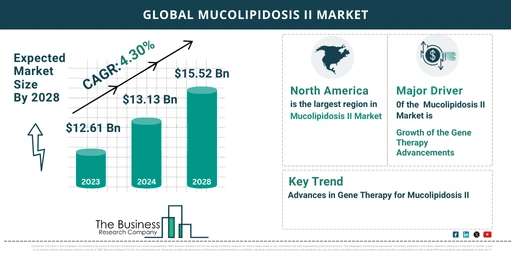
- 2023 Market Size: $12.61 billion.
- 2024 Forecast: Expected to grow to $13.13 billion.
- CAGR: 4.1% from 2023 to 2024.
Key Drivers of Growth
- Increasing Regulatory Approvals: More orphan drugs receive the green light.
- Rise in Disease Awareness: Public and healthcare professionals become more informed.
- Surge in Clinical Trials: More trials lead to better treatments.
- Growing Genetic Testing: Early detection aids in timely treatment.
- Increased Funding for Rare Diseases: Governments and organizations boost research.
- Enhanced Diagnosis Rates: More patients are correctly diagnosed.
Future Market Projections
- 2028 Market Size: Expected to reach $15.52 billion.
- CAGR: 4.3% from 2024 to 2028.
View More On The Mucolipidosis II Market Report 2024 – https://www.thebusinessresearchcompany.com/report/mucolipidosis-ii-global-market-report
Drivers for Future Growth
- Rising Awareness: Healthcare professionals and patients become more knowledgeable.
- Boost in R&D Activities: More innovation in treatments and diagnostics.
- Emerging Biomarkers: Early diagnosis becomes more accurate.
- Investment in Rare Disease Therapies: Companies focus on niche markets.
- Improved Reimbursement Policies: Patients can access treatments more easily.
- Growing Demand for Gene Therapy: This promising treatment option gains traction.
Trends Shaping the Market
- Advancements in AI in Medicine: AI improves diagnosis and treatment options.
- Innovations in Genetic Testing: New technologies enhance precision.
- Improved Diagnostic Methods: Early detection becomes more reliable.
- Progress in Therapeutic Treatments: New treatments offer better outcomes.
- Growth of Telemedicine: Patients receive care remotely, expanding access.
Gene Therapy Advancements Propel Market Growth
- Importance of Gene Therapy:
- Core Function: Corrects faulty genes or introduces new ones.
- Application to Mucolipidosis II: Addresses the root genetic cause.
- Gene Therapy Techniques: Gene addition, editing, silencing, and regulation.
- Recent Developments:
- Example: In April 2024, the American Society of Gene & Cell Therapy reported a 10% increase in phase III clinical trials for gene therapies.
- Impact: Gene therapy is expected to be a key driver of market growth.
Impact of Increased R&D Activities
- Importance of R&D:
- Objective: Discover new knowledge, products, and processes.
- Focus Areas: Understanding of mucolipidosis II, developing gene therapies, and improving delivery methods.
- Examples:
- UK Government Investment: In 2022, research and development spending increased to £15.5 billion ($19.67 billion), a 10.5% rise from 2021.
- Impact: Continuous R&D efforts are expected to propel the market forward.
Leading Companies in the Mucolipidosis II Market
- Major Players:
- Pfizer Inc., Merck & Co. Inc., Sanofi S.A., Novartis AG, Takeda Pharmaceutical Company Limited.
- Teva Pharmaceutical Industries Ltd., Sun Pharmaceutical Industries Limited, Jazz Pharmaceuticals, Cipla Limited.
- Lupin Limited, Zydus Lifesciences Limited, Alembic Pharmaceuticals, Ultragenyx Pharmaceutical Inc.
- Regenxbio Inc., Denali Therapeutics Inc., Mylan N.V., Intellia Therapeutics Inc., Avrobio Inc.
- Orchard Therapeutics plc, Eloxx Pharmaceuticals Inc., Lysogene S.A., Sio Gene Therapies.
- Homology Medicines Inc., BSN medical GmbH, Abeona Therapeutics Inc.
Advances in Gene Therapy for Mucolipidosis II
- Focus on Gene Therapy:
- Objective: Correct metabolic dysfunctions by delivering functional genes.
- Recent Developments:
- M6P Therapeutics: Received orphan drug designation for its M002 S1S3 PTase AAV gene therapy in February 2021.
- Mechanism: Uses an AAV-9 vector to deliver a truncated phosphotransferase (PTase) enzyme, restoring function in transduced cells within three weeks.
- Impact: These innovations are expected to significantly advance the treatment of mucolipidosis II.
Market Segmentation
- By Treatment:
- Categories: Antibiotics, Physical Therapy, Hip Replacement, Experimental Therapies, Other Treatments.
- By Mode of Administration:
- Types: Injectable, Oral, Other Modes.
- By Symptoms:
- Conditions: Deafness, Hypotonia, Abnormal Spine Curvature, Mental Retardation, Low Motor Skills Growth, Other Symptoms.
- By End-User:
- Sectors: Hospitals, Homecare, Specialty Clinics, Other End-Users.
Geographic Insights
- Largest Region: North America led the market in 2023.
- Growth Potential: Further growth is expected in various global regions due to increasing awareness and advancements in treatment options.
The mucolipidosis II market is on a steady growth trajectory, driven by advancements in gene therapy, research and development activities, and increased awareness. As innovations continue to emerge, particularly in gene therapy, the market is poised for further expansion.
Request A Sample Of The Global Mucolipidosis II Market Report 2024:
https://www.thebusinessresearchcompany.com/sample_request?id=16746&type=smp