Key Insights On The Viral Vector Manufacturing Market 2024 – Size, Driver, And Major Players
The Business Research Company’s global market reports are now updated with the latest market sizing information for the year 2024 and forecasted to 2033
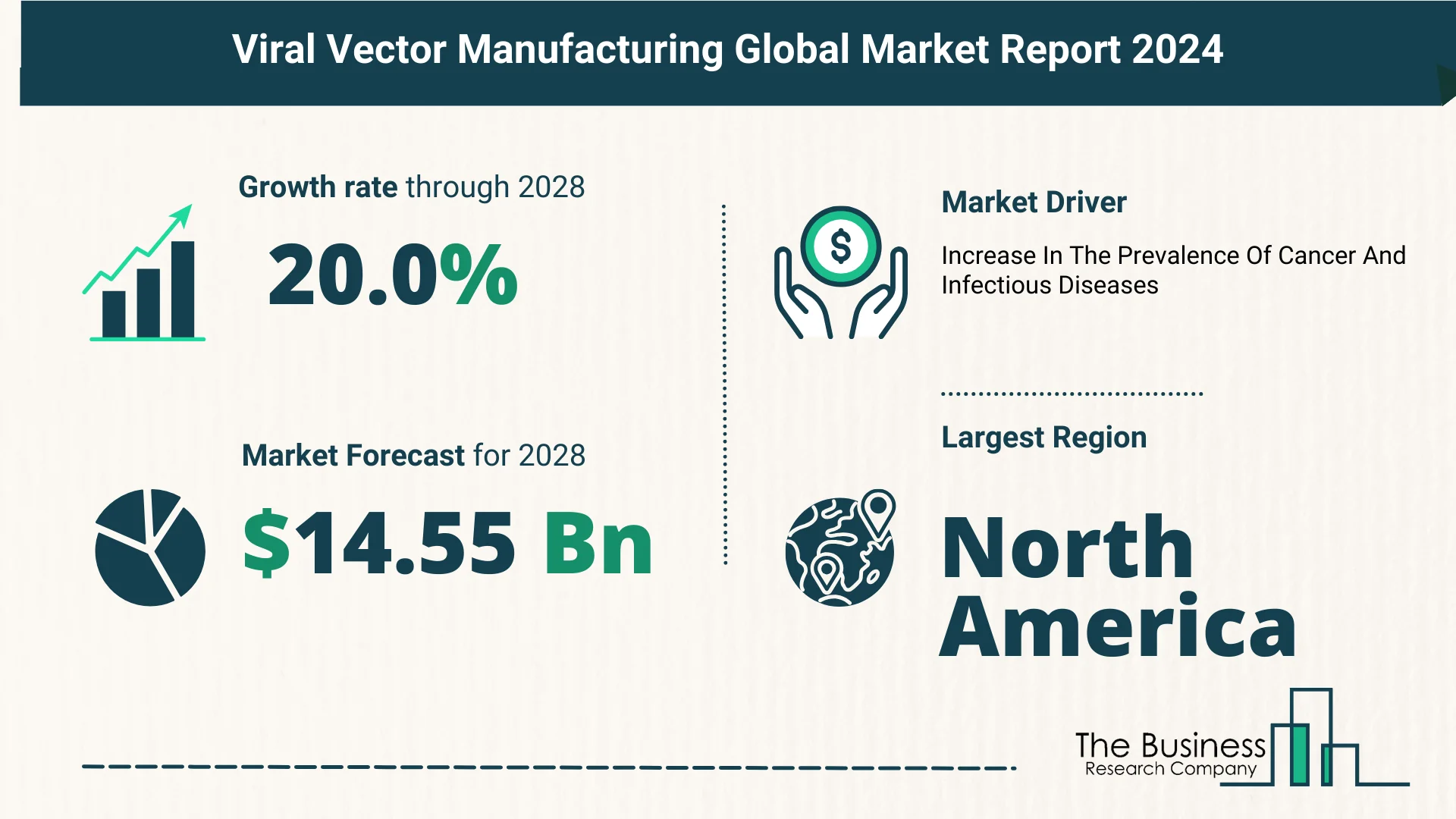
As per The Business Research Company’s Viral Vector Manufacturing Global Market Report 2024, the viral vector manufacturing market is expected to show significant growth in the forecast period.
The viral vector manufacturing market has witnessed exponential growth in recent years, with promising forecasts indicating further expansion. Let’s delve into the factors driving this growth and the trends shaping the market landscape.
Exponential Growth and Projections:
- The market size surged from $5.82 billion in 2023 to $7 billion in 2024, boasting a remarkable compound annual growth rate (CAGR) of 20.4%.
- Projections suggest a continued upward trajectory, with expectations of reaching $14.55 billion by 2028 at a CAGR of 20.0%.
Drivers of Growth:
- Pipeline of Gene Therapies: The burgeoning pipeline of gene therapies has been a significant contributor to market expansion.
- Clinical Success and Approvals: Increasing clinical successes and regulatory approvals have fueled market growth.
- Investment in Biopharmaceuticals: Heightened investment in biopharmaceuticals has propelled the demand for viral vectors.
- Prevalence of Genetic Disorders: Rising prevalence of genetic disorders has necessitated advancements in viral vector manufacturing.
View More On The Viral Vector Manufacturing Market Report 2024 – https://www.thebusinessresearchcompany.com/report/viral-vector-manufacturing-global-market-report
Impact of Rising Cancer and Infectious Diseases:
- Prevalence Surge: The rise in cancer and infectious diseases worldwide is a primary catalyst for market growth.
- Applications in Oncology: Viral vectors are increasingly utilized in oncology for tumor antigen production and immune response elicitation.
- Vaccine Development: Viral vectors play a crucial role in vaccine development against infectious diseases.
- Clinical Trials and Commercialization: Continued clinical trials and commercialization efforts drive market expansion.
Product Innovation Driving Market Dynamics:
- Introduction of Innovative Platforms: Major players are introducing innovative platforms to streamline viral vector manufacturing processes.
- Merck’s VirusExpress 293 AAV Production Platform: Merck’s platform aims to expedite process development and clinical manufacture, fostering market growth.
Thermo Fisher Scientific’s Strategic Acquisition:
- Expansion Initiatives: Thermo Fisher Scientific’s acquisition of Henogen S.A. aims to bolster global biomanufacturing capabilities, particularly in cell and gene therapies and vaccines.
Market Segmentation:
- Type: Segmented into Adenoviral Vectors, Adeno-Associated Viral Vectors, Lentiviral Vectors, Retroviral Vectors, and Other Types.
- Disease: Categorized into Cancer, Genetic Disorders, Infectious Diseases, and Other Diseases.
- Workflow: Segmented into Upstream Processing and Downstream Processing.
- Application: Includes Gene and Cell Therapy Development, Vaccine Development, Biopharmaceutical and Pharmaceutical Discovery, and Biomedical Research.
- End-User: Encompasses Research Organizations, Biotech and Pharmaceutical Companies, and Others.
Regional Insights:
- North America: Emerged as the largest region in the viral vector manufacturing market in 2023.
- Asia-Pacific: Expected to witness the fastest growth in the forecast period, highlighting the global expansion of manufacturing facilities.
Conclusion: The viral vector manufacturing market’s remarkable growth trajectory is underpinned by factors such as technological innovations, rising disease prevalence, and strategic initiatives by key players. As the market continues to evolve, collaborations, regulatory compliance, and product innovations will remain pivotal in shaping its future landscape.
Request A Sample Of The Global Viral Vector Manufacturing Market Report 2024:
https://www.thebusinessresearchcompany.com/sample_request?id=12398&type=smp