How is the Viral Vectors And Plasmid DNA Manufacturing Market Poised for Growth: Trends and Opportunities Through 2034
Updated 2025 Market Reports Released: Trends, Forecasts to 2034 – Early Purchase Your Competitive Edge Today!
How has the viral vectors and plasmid dna manufacturing market grown over the years?
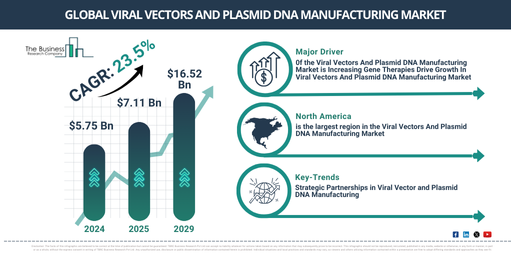
In recent years, there has been a significant expansion in the market size for the manufacturing of viral vectors and plasmid DNA. It is projected to escalate from a value of $5.75 billion in 2024 to $7.11 billion in 2025, demonstrating a compound annual growth rate (CAGR) of 23.8%. This surge during the historic period is linked to factors such as the rising demand for personalized treatment, advancements in vaccine development, the accessibility of capital for the progression of gene therapy, the effectiveness of viral vectors in the delivery of gene therapy, and their prospective uses in avant-garde drug delivery techniques.
What Is the forecasted market size and growth rate for the viral vectors and plasmid dna manufacturing market?
The market for viral vectors and plasmid DNA manufacturing is projected to witness a substantial expansion in the following years, with an expected value of $16.52 billion by 2029 and a predicted CAGR of 23.5%. This projected growth within the forecasted period is related to factors such as the growing number of gene therapy applications, expanding market players, surging incidence of infectious diseases and genetic disorders, an increase in the funding for cell and gene therapy research, and a rising prevalence of target diseases and disorders. The forecast period is also expected to witness major market trends including technological progression, enhancements in gene therapy, the adoption of cutting-edge technologies, the shift towards single-use technologies, and improvements in vaccine technology.
Get your viral vectors and plasmid dna manufacturing market report here!
What are the major factors driving growth in the viral vectors and plasmid dna manufacturing market?
The viral vectors and plasmid DNA manufacturing market’s growth is likely to be fueled by the rising number of gene therapies. Gene therapy, which involves the modification, introduction, or deletion of genetic material within an individual’s cells to prevent or treat illnesses, is crucial for progression. This is largely due to increased funds poured into biotechnology research and development, coupled with an improved understanding of genetic and cellular disease mechanisms. Viral vectors and plasmid DNA manufacturing play an indispensable role in delivering therapeutic genes into patients’ cells, thereby tackling genetic disorders at a molecular level. As identified by the American Society of Gene & Cell Therapy, a US-based non-profit organization, there was a 10% increase in Phase III clinical trials of gene therapies in Q3 2023, marking the first quarterly rise since Q3 2022. As such, the escalating number of gene therapies is catalyzing the growth of the viral vectors and plasmid DNA manufacturing market.
What key areas define the segmentation of the global viral vectors and plasmid dna manufacturing Market?
The viral vectors and plasmid dna manufacturing market covered in this report is segmented –
1) By Product Type: Plasmid Deoxyribonucleic Acid (DNA), Viral Vector, Non-Viral Vector
2) By Scale Of Operation: Preclinical, Clinical, Commercial
3) By Therapeutic Area: Oncological Disorders, Rare Diseases, Neurological Disorders, Sensory Disorders, Metabolic Disorders, Musculoskeletal Disorders, Blood Disorders, Immunological Disorders, Other Disorders
4) By Application Area: Gene Therapies, Cell Therapies, Vaccines
5) By Type Of Manufacturer: In-house Manufacturers, Contract Manufacturing Organizations
Subsegments:
1) By Plasmid Deoxyribonucleic Acid (DNA): Linear Plasmid DNA, Circular Plasmid DNA, High-Copy Plasmid DNA, Low-Copy Plasmid DNA
2) By Viral Vector: Adenoviral Vectors, Lentiviral Vectors, Adeno-Associated Viral Vectors (AAV), Retroviral Vectors
3) By Non-Viral Vector: Liposome-Based Vectors, Polymer-Based Vectors, Nanoparticle-Based Vectors, Electroporation-Based Vectors
Get your free sample now – explore exclusive market insights:
https://www.thebusinessresearchcompany.com/sample.aspx?id=20690&type=smp
What are the top market players propelling the growth of the viral vectors and plasmid dna manufacturing industry?
Major companies operating in the viral vectors and plasmid DNA manufacturing market are Merck KGaA, Thermo Fisher Scientific Inc., Astellas Pharma Inc., Lonza Group AG, Catalent Inc., Revvity Inc., Wuxi Biologics Inc., BioMarin Pharmaceutical, FUJIFILM Diosynth Biotechnologies, Miltenyi Biotec GmbH, Aldevron LLC, Takara Bio Inc., Poseida Therapeutics Inc., RegenxBio Inc., Cobra Biologics, Finvector Vision Therapies Limited, Batavia Biosciences B.V., uniQure N.V., Addgene Inc., BioNTech IMFS GmbH, Creative Biogene Inc., Genezen Laboratories Inc., Virovek Incorporation, Waisman Biomanufacturing, Axplora GmbH
What are the key trends shaping the future of the viral vectors and plasmid dna manufacturing market?
Prominent firms in the viral vectors and plasmid DNA manufacturing sector are prioritizing strategic alliances to increase their production capacities, improve technological competence, and speed up the development and global launch of gene therapies. These partnerships allow companies to pool their resources and infrastructure, thereby augmenting their production capacity to satisfy the rising need for gene therapies. For example, in June 2024, Charles River Laboratories International Inc., an American preclinical and clinical contract research company, and Captain T Cell, a spin off from the acclaimed Max Delbrück Center Berlin, Germany, initiated a plasmid DNA and retrovirus vector production program agreement. This association grants Captain T Cell access to proven contract development and manufacturing (CDMO) capabilities and consultation facilities. It also has the objective of hastening the transition from preclinical research to clinical trials for an innovative TCR-T cell therapy aimed at solid tumors.
Unlock exclusive market insights – purchase your research report now for a swift delivery!
https://www.thebusinessresearchcompany.com/purchaseoptions.aspx?id=20690
What regions are dominating the viral vectors and plasmid dna manufacturing market growth?
North America was the largest region in the viral vectors and plasmid DNA manufacturing market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the viral vectors and plasmid DNA manufacturing market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa.
Browse Through More Similar Reports By The Business Research Company:
Viral Vectors & Plasmid DNA Global Market Report 2025
https://www.thebusinessresearchcompany.com/report/viral-vectors-and-plasmid-dna-global-market-report
Bacterial And Plasmid Vectors Global Market Report 2025
https://www.thebusinessresearchcompany.com/report/bacterial-and-plasmid-vector-global-market-report
Prenatal DNA Sequencing Global Market Report 2025
https://www.thebusinessresearchcompany.com/report/prenatal-dna-sequencing-global-market-report
About The Business Research Company:
With over 15000+ reports from 27 industries covering 60+ geographies, The Business Research Company has built a reputation for offering comprehensive, data-rich research and insights. Armed with 1,500,000 datasets, the optimistic contribution of in-depth secondary research, and unique insights from industry leaders, you can get the information you need to stay ahead in the game.
Contact us at:
The Business Research Company: https://www.thebusinessresearchcompany.com/
Americas +1 3156230293
Asia +44 2071930708
Europe +44 2071930708
Email us at info@tbrc.info
Follow us on:
LinkedIn: https://in.linkedin.com/company/the-business-research-company
YouTube: https://www.youtube.com/channel/UC24_fI0rV8cR5DxlCpgmyFQ
Global Market Model: https://www.thebusinessresearchcompany.com/global-market-model
Found this article helpful? Share it on: